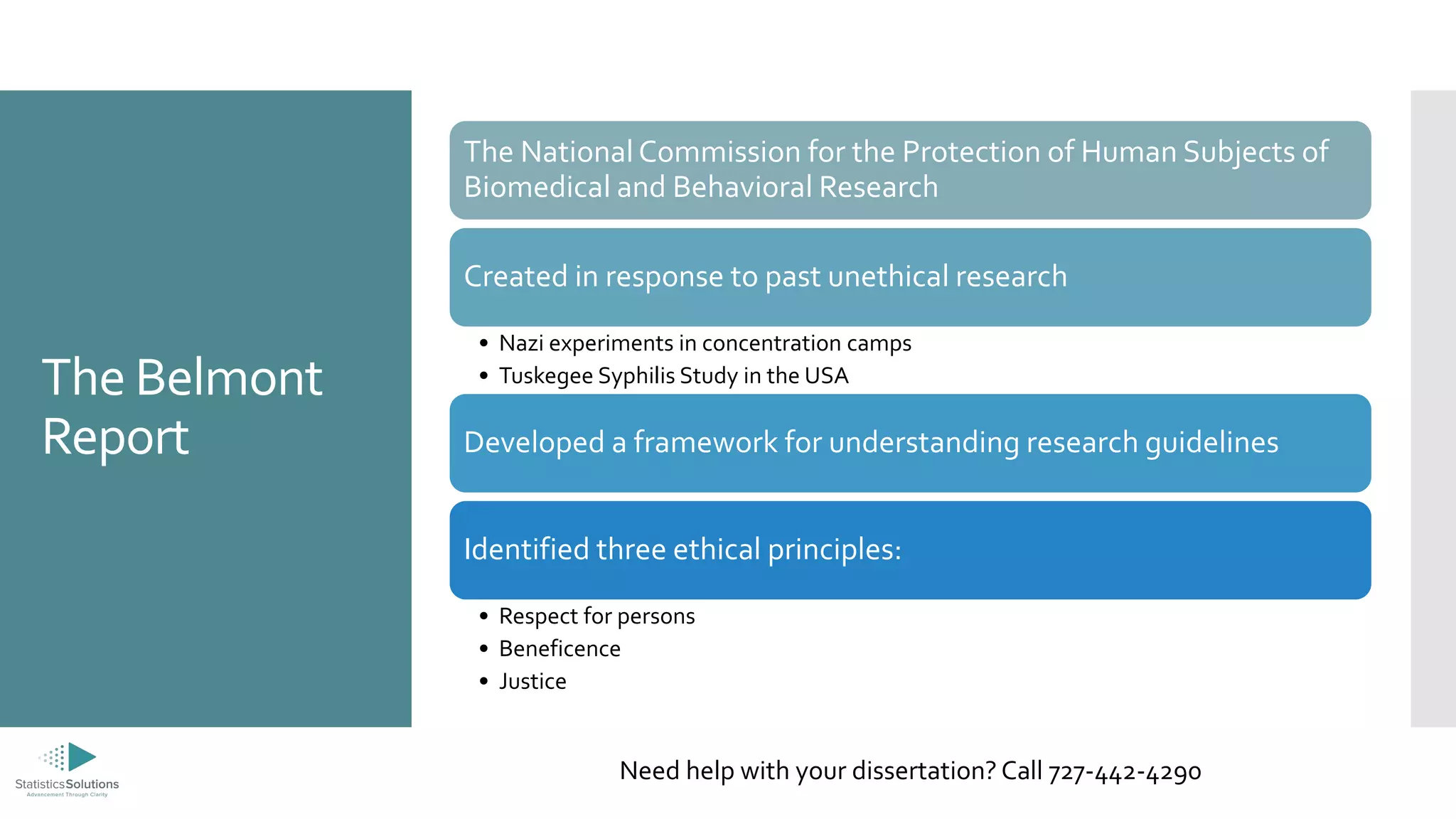
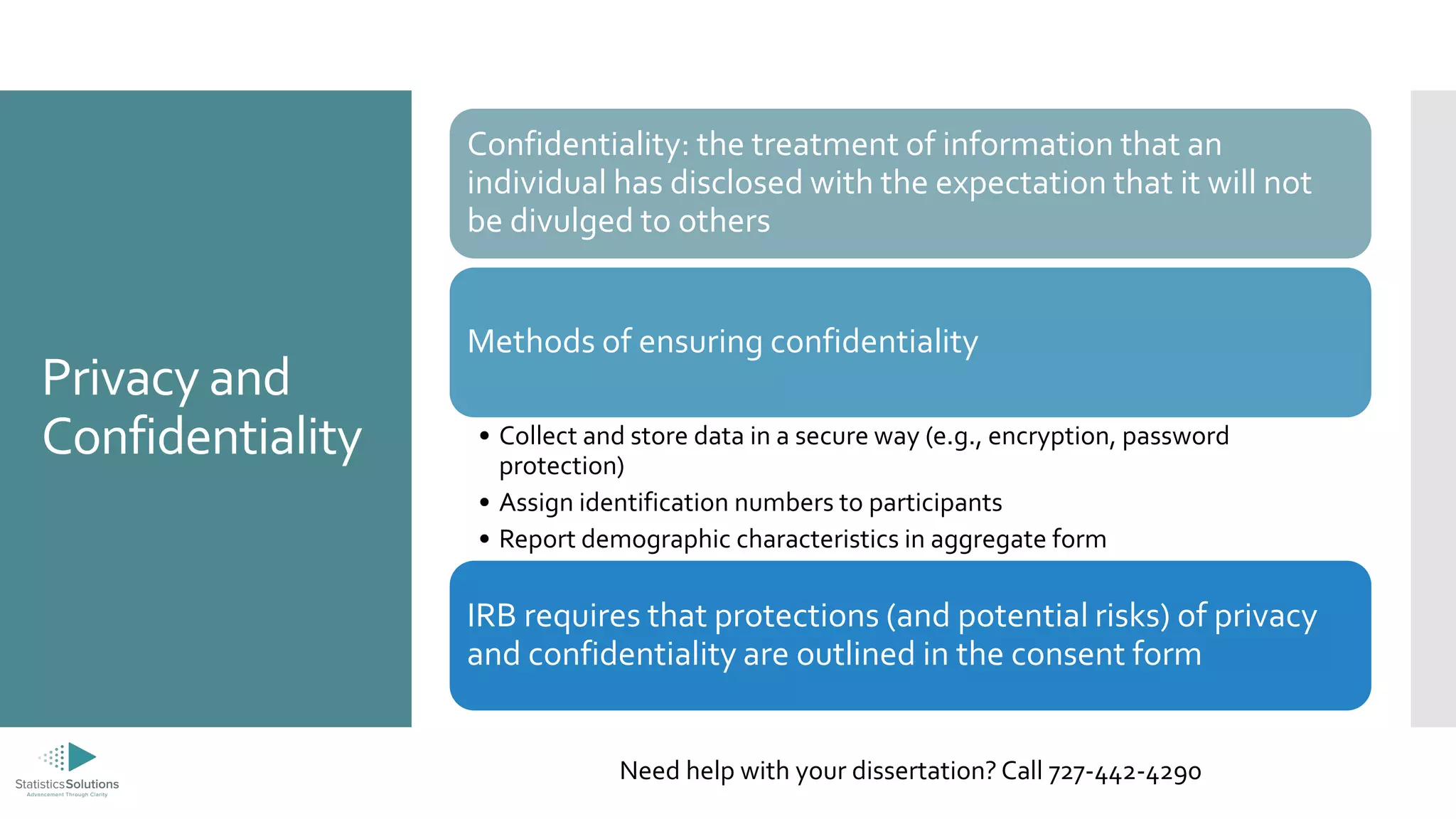
The document provides an overview of the ethical principles and processes related to Institutional Review Boards (IRBs) in research involving human subjects, emphasizing respect for persons, beneficence, and justice. It details the informed consent process, privacy and confidentiality issues, and categorizes risk levels associated with research reviews. Additionally, it offers information about dissertation consulting services, including a free consultation option.