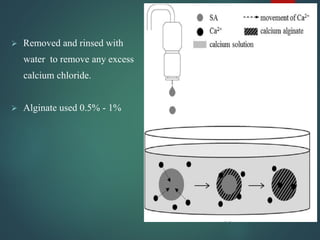
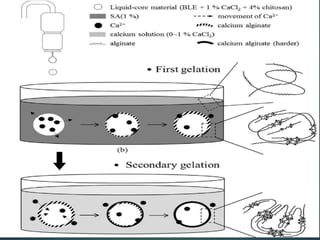
This document summarizes a seminar presentation on spherification in molecular gastronomy. Spherification is a technique introduced by Ferran Adria in 2003 to turn liquids into spheres using sodium alginate and calcium chloride. The presentation defines molecular gastronomy and discusses the basic chemistry behind spherification reactions. It also describes different spherification methods, factors that affect the process, equipment used like a spherificator, applications, examples like strawberry caviar, and the future scope of spherification.