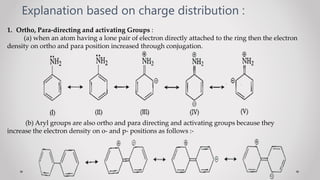
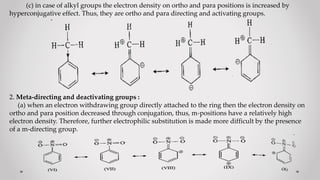
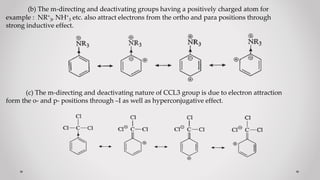
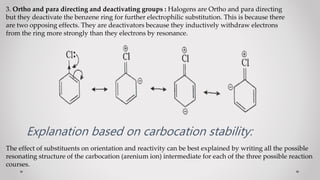
1. When a monosubstituted benzene undergoes electrophilic substitution, the position of the incoming group (orientation) and reaction rate (reactivity) are determined by the original substituent.
2. Substituents are classified as ortho/para-directing and activating, meta-directing and deactivating, or ortho/para-directing and deactivating.
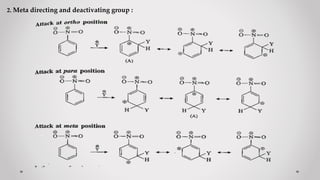
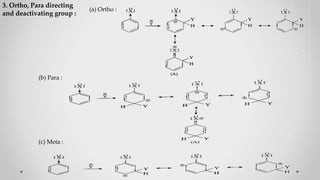
3. Orientation and reactivity can be explained by resonance structures of possible carbocation intermediates that form during the reaction. The most stable carbocation will determine the preferred position for incoming groups.