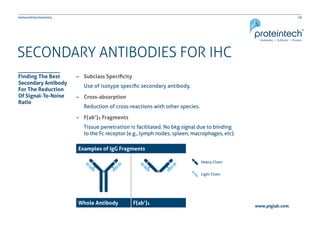
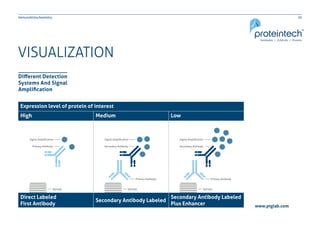
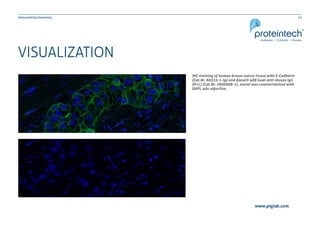
The document provides a comprehensive guide on immunohistochemistry (IHC), detailing its principles, applications, and procedural protocols for visualizing proteins in tissue samples. It covers key aspects such as antigen retrieval methods, antibody selection, visualization techniques, and troubleshooting tips to optimize the IHC process. This resource is aimed at aiding researchers and laboratory technicians in effectively utilizing IHC in medical diagnostics and research.