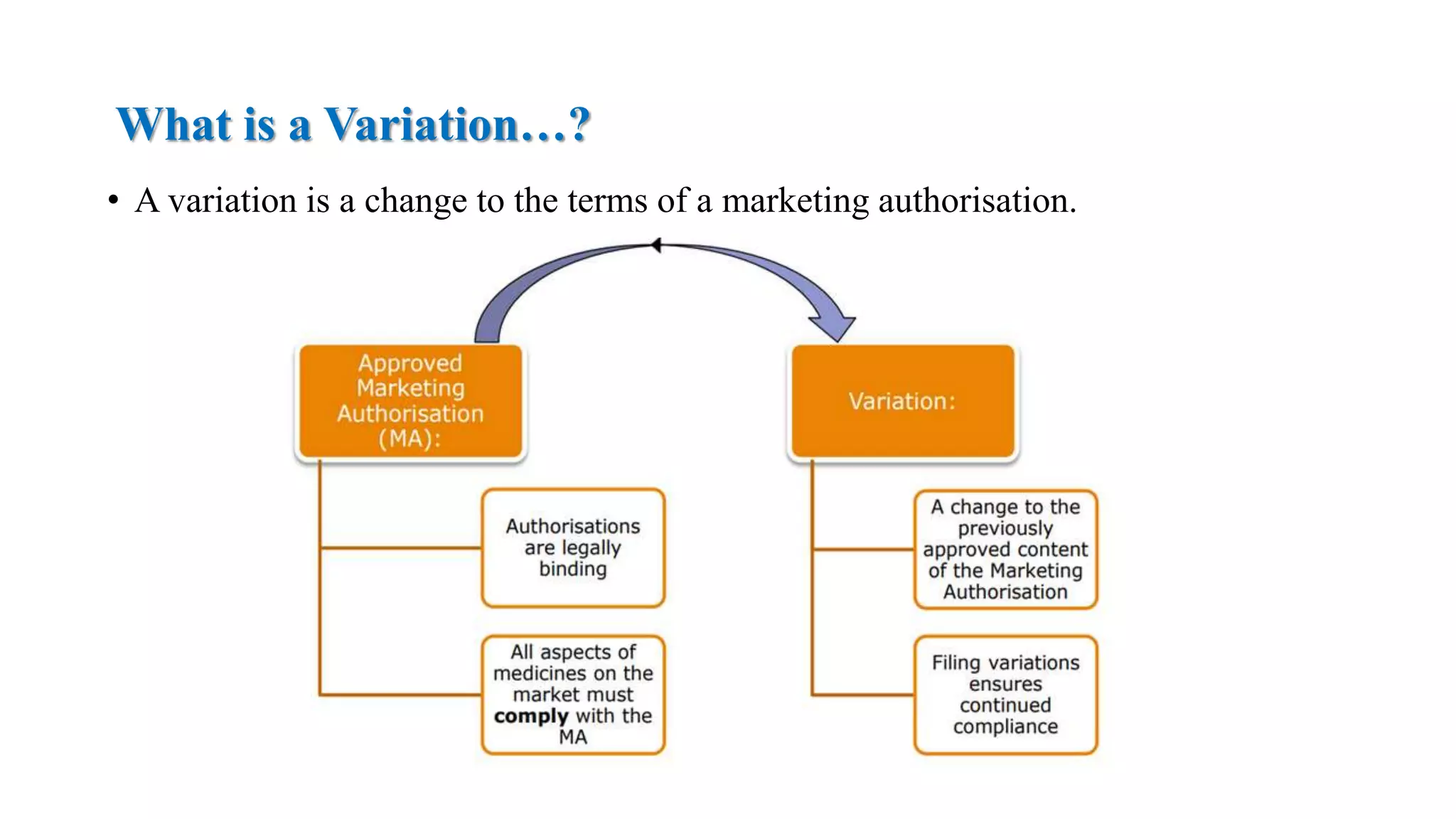
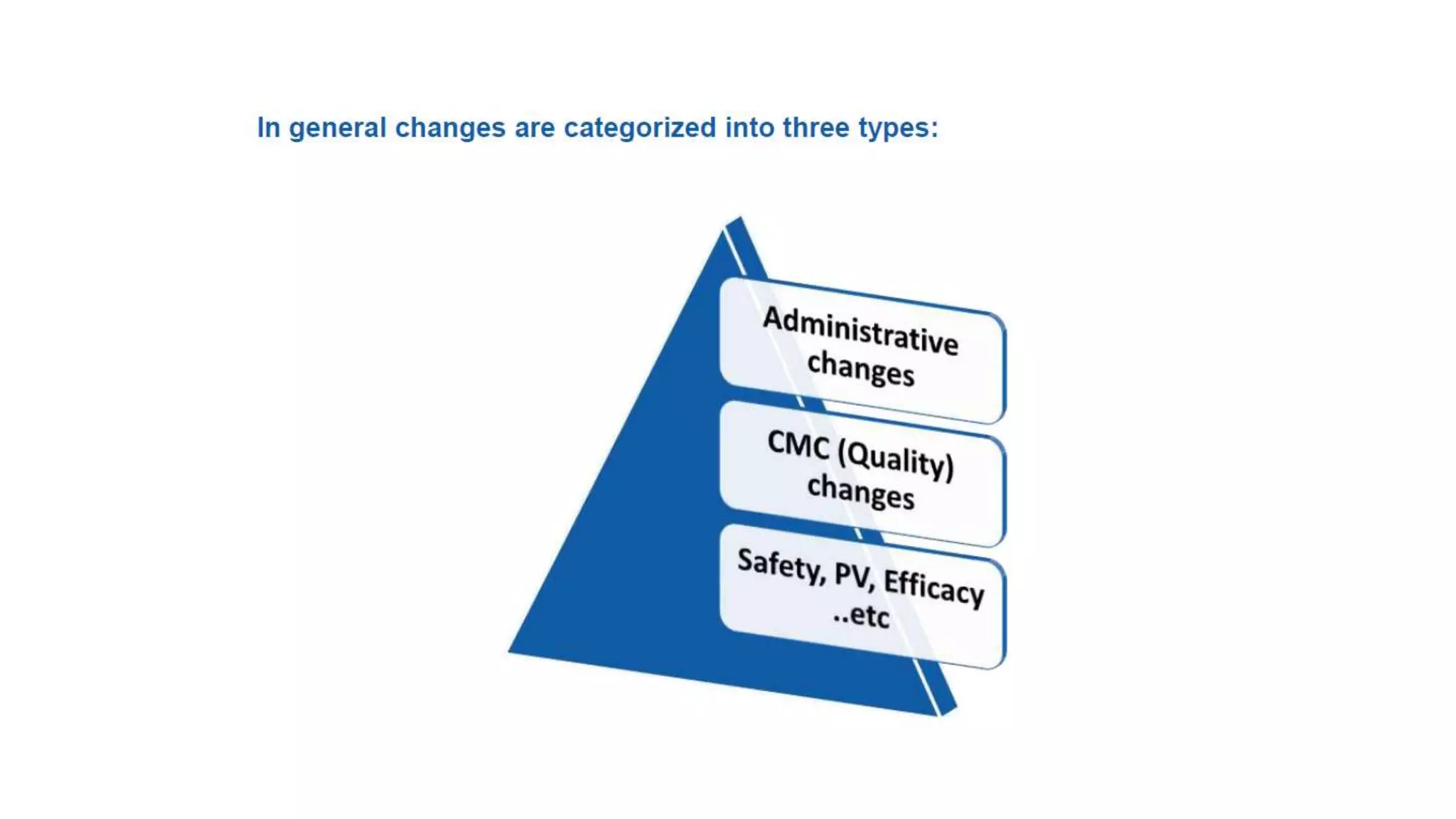
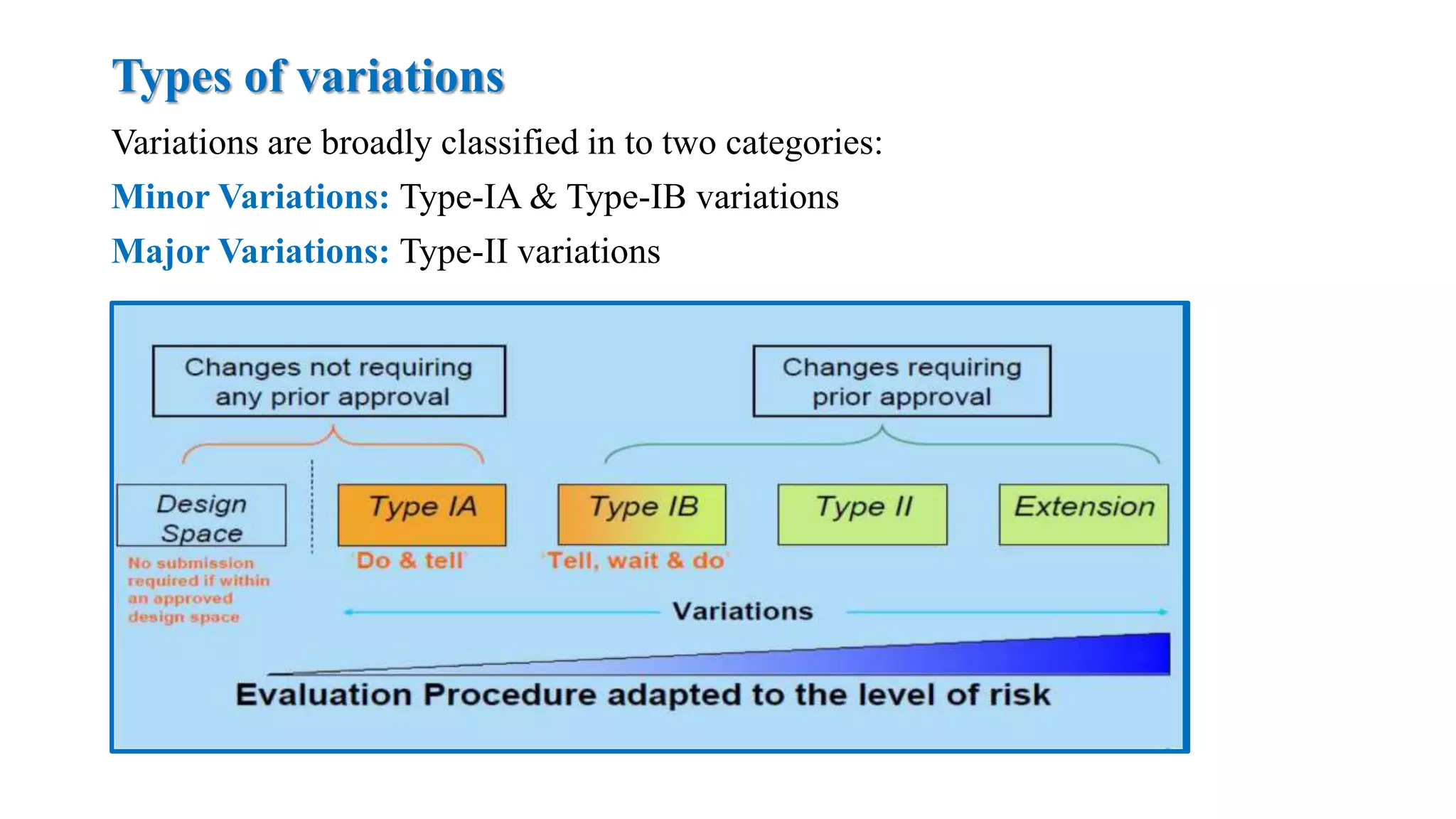
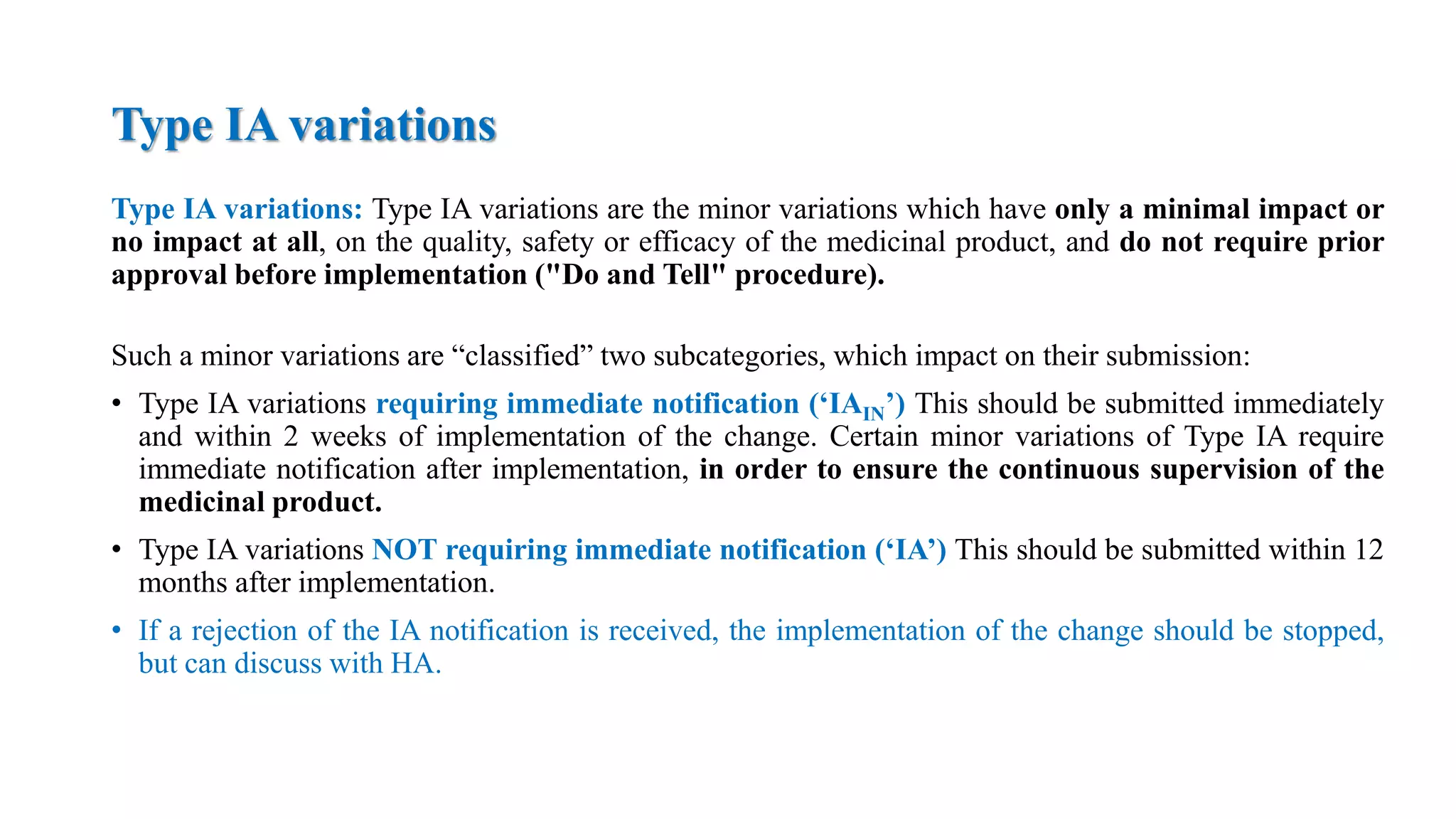
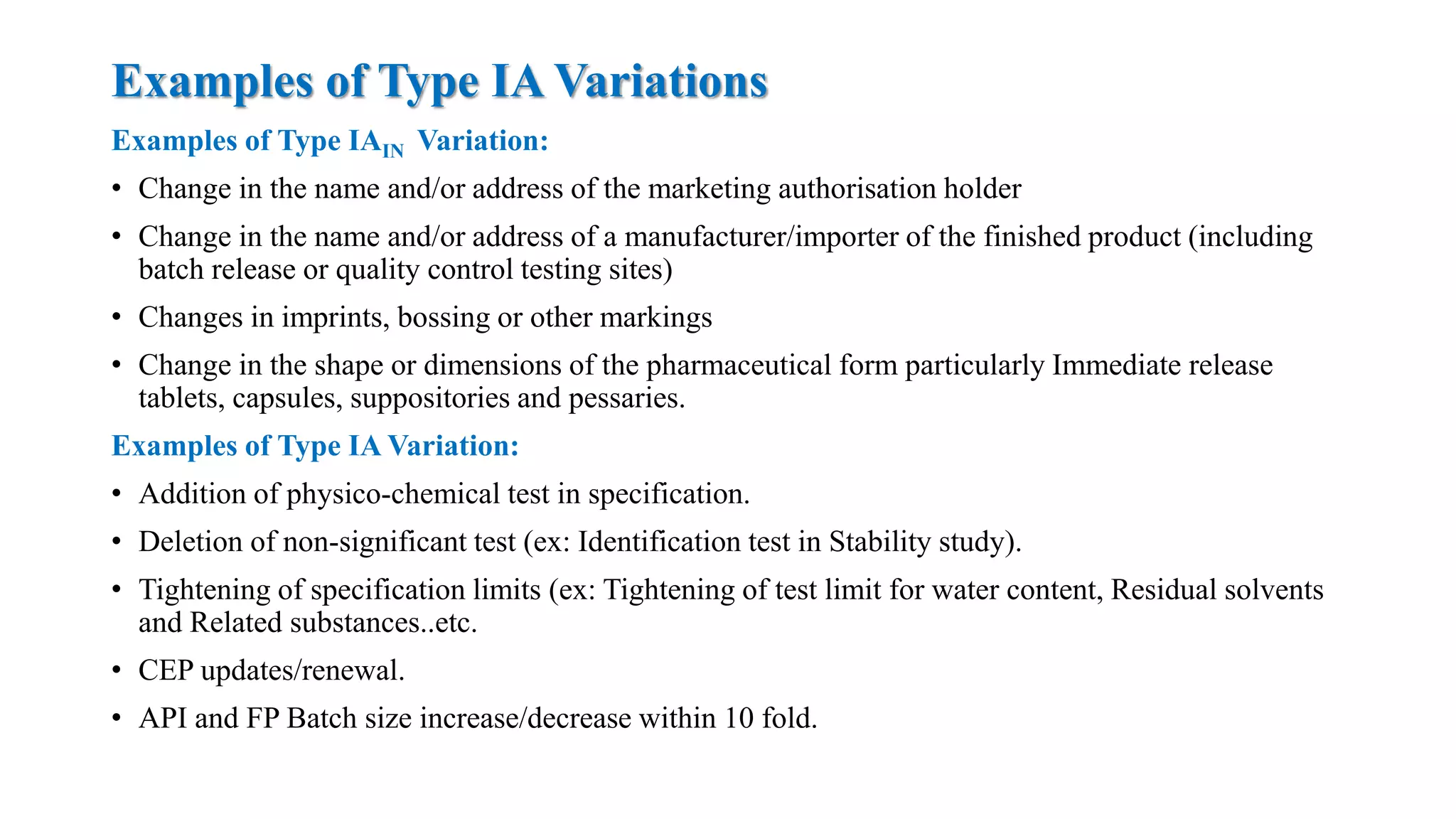
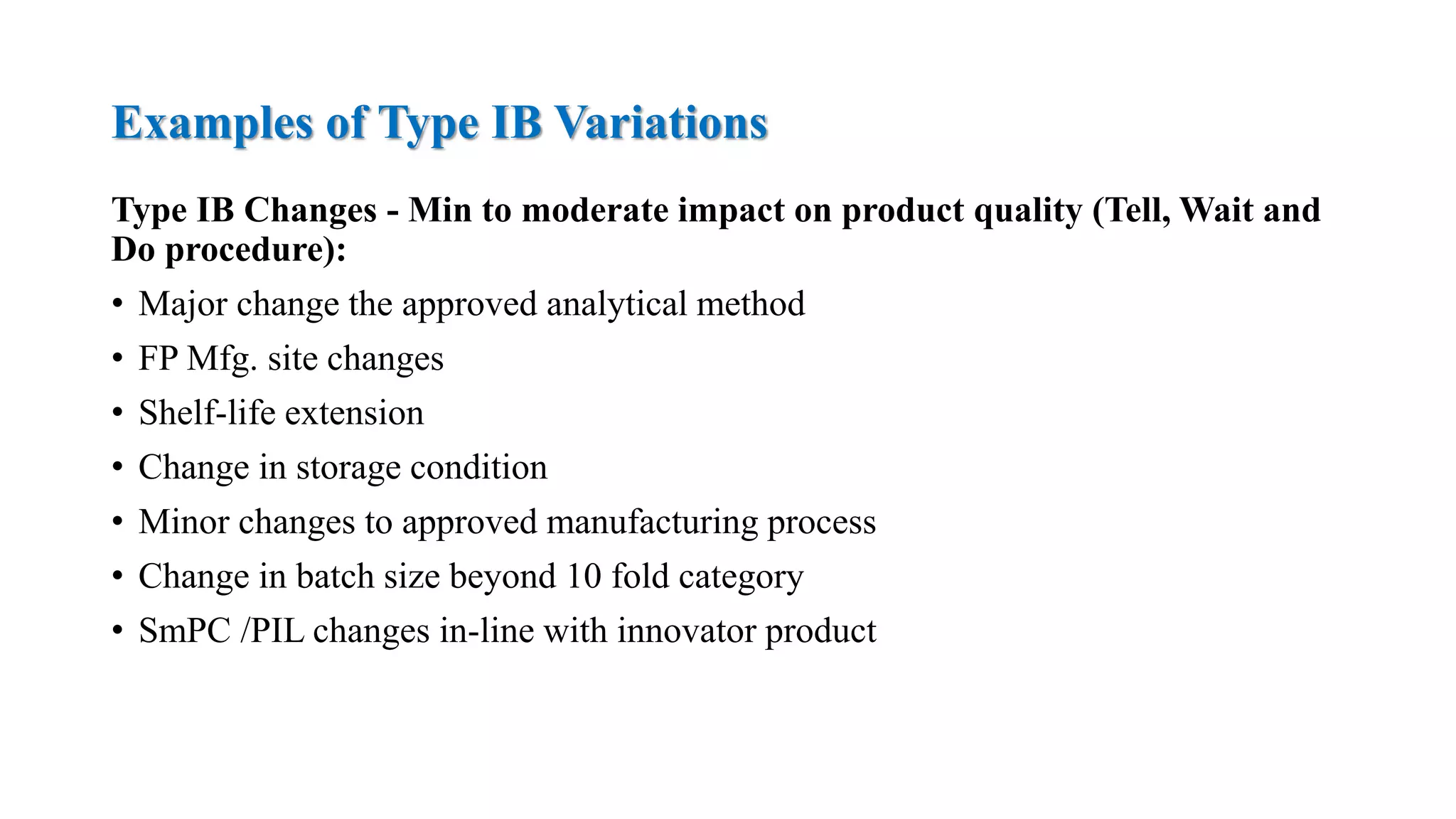
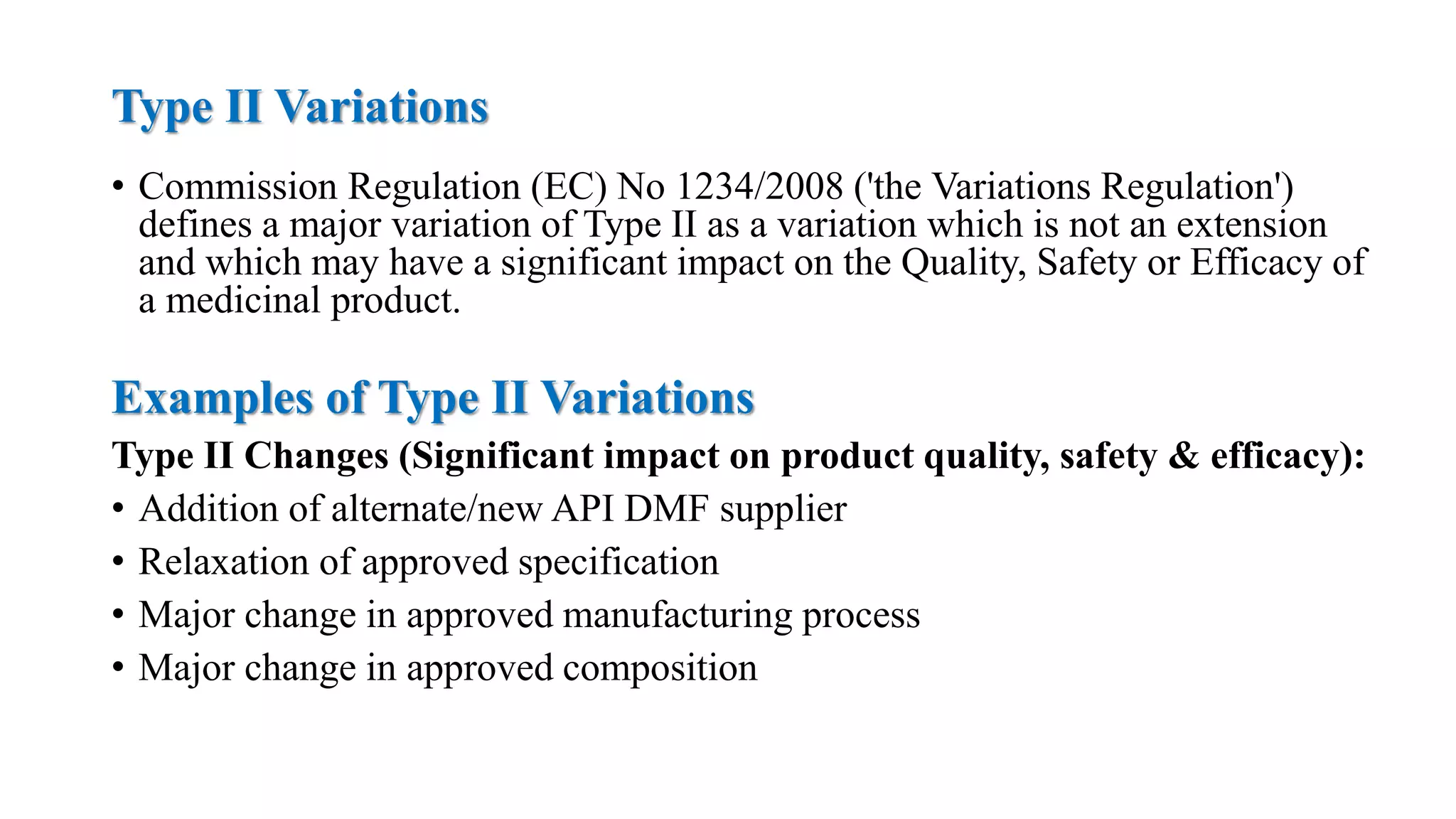
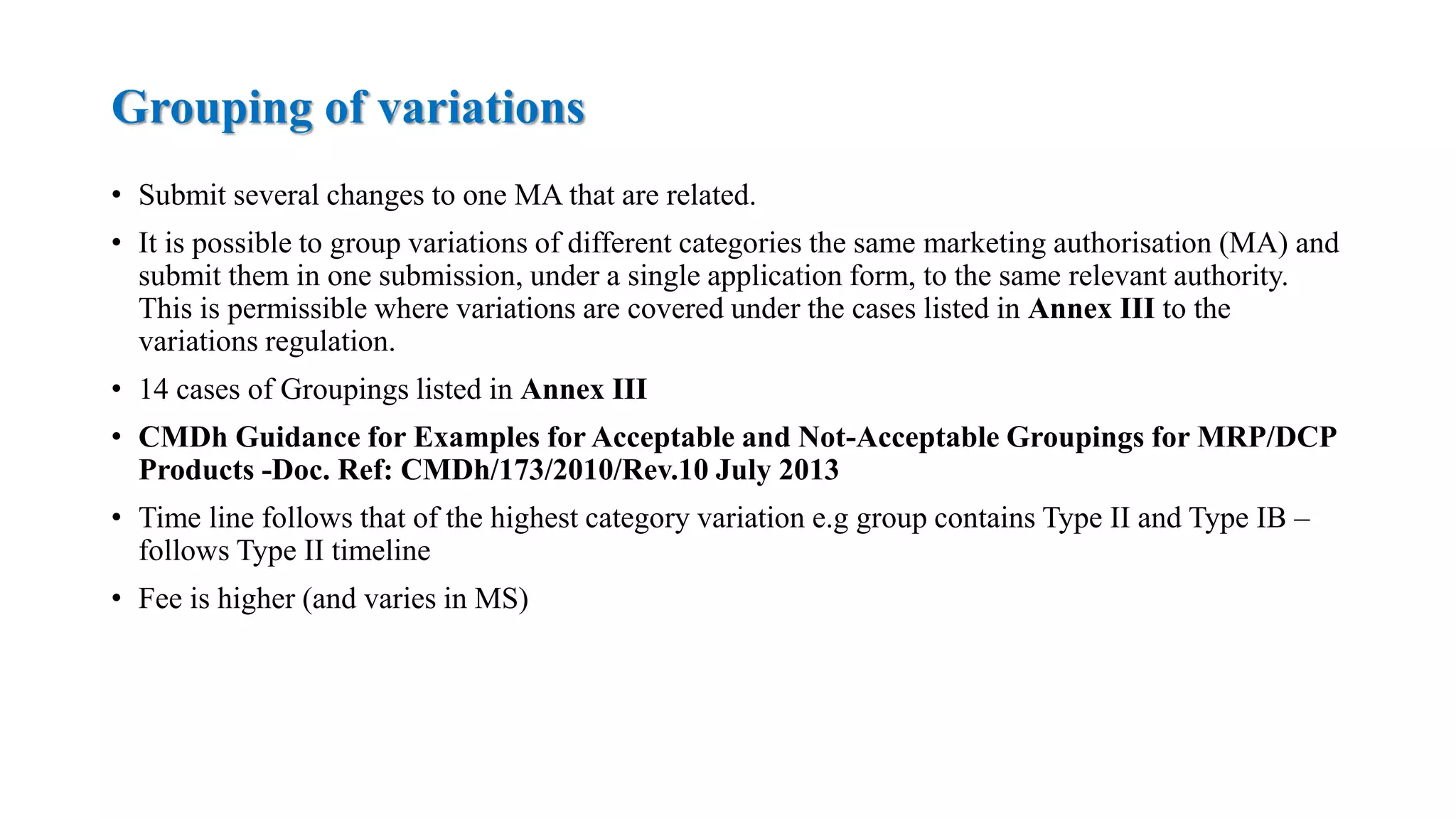
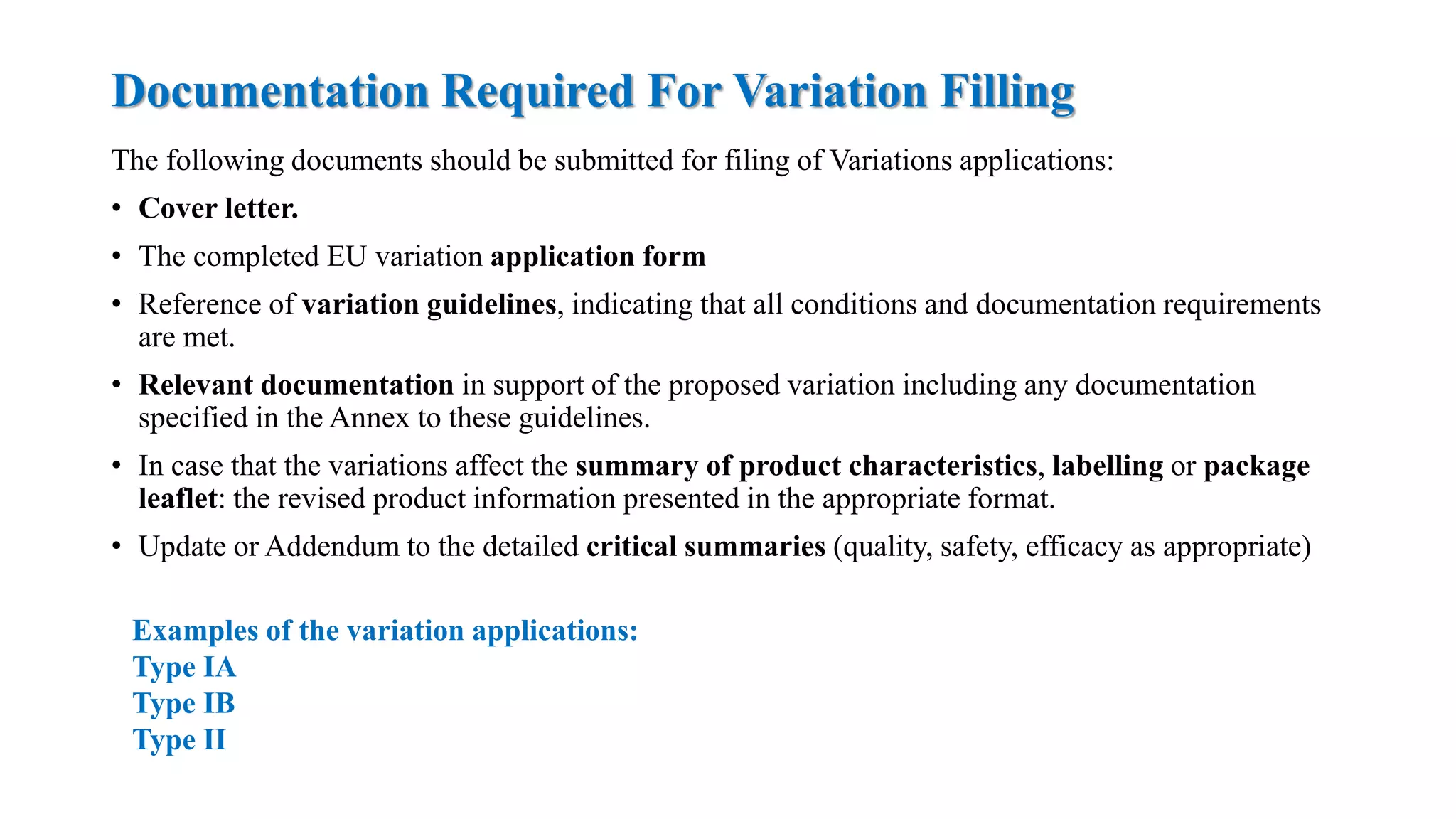
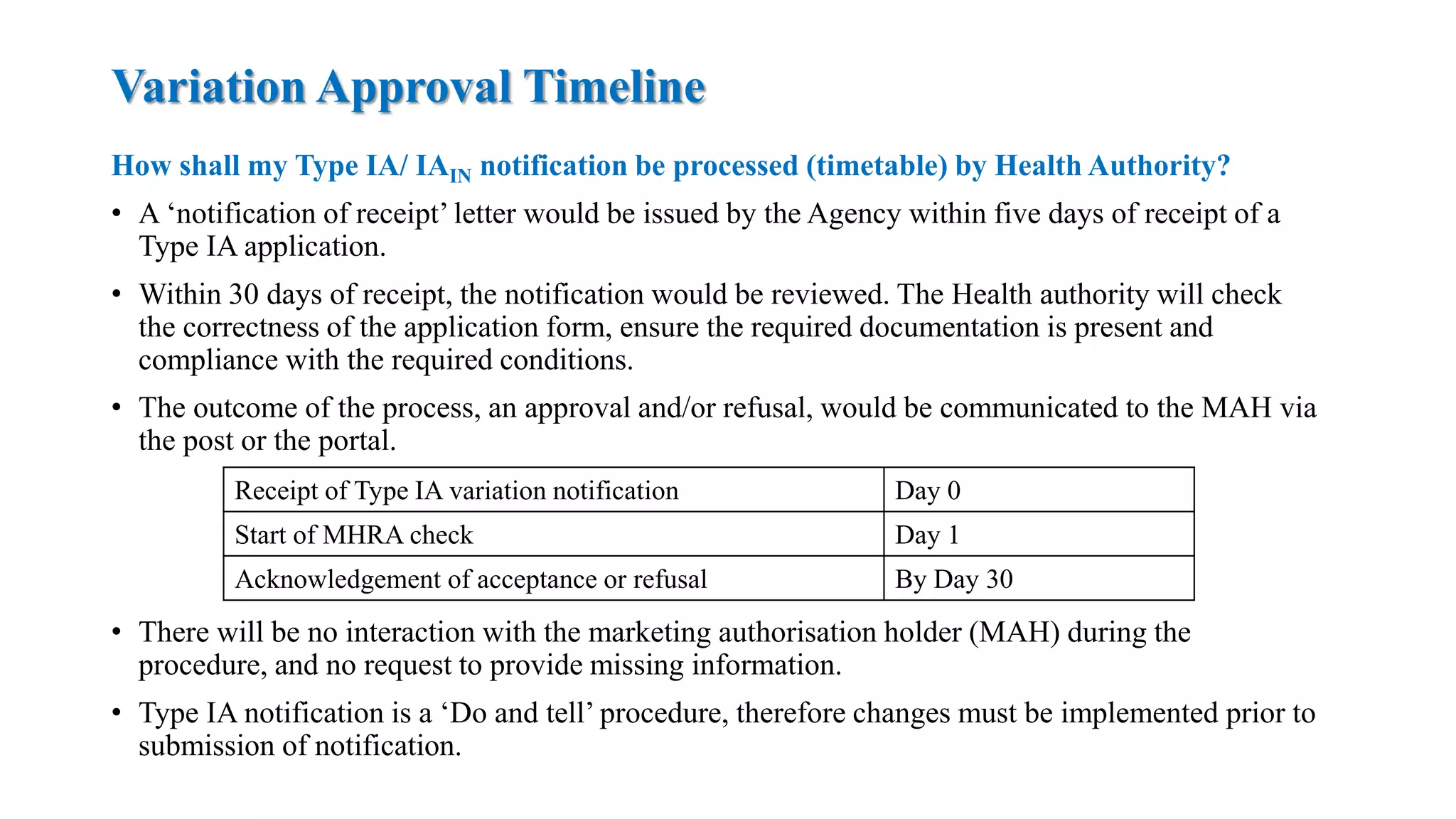
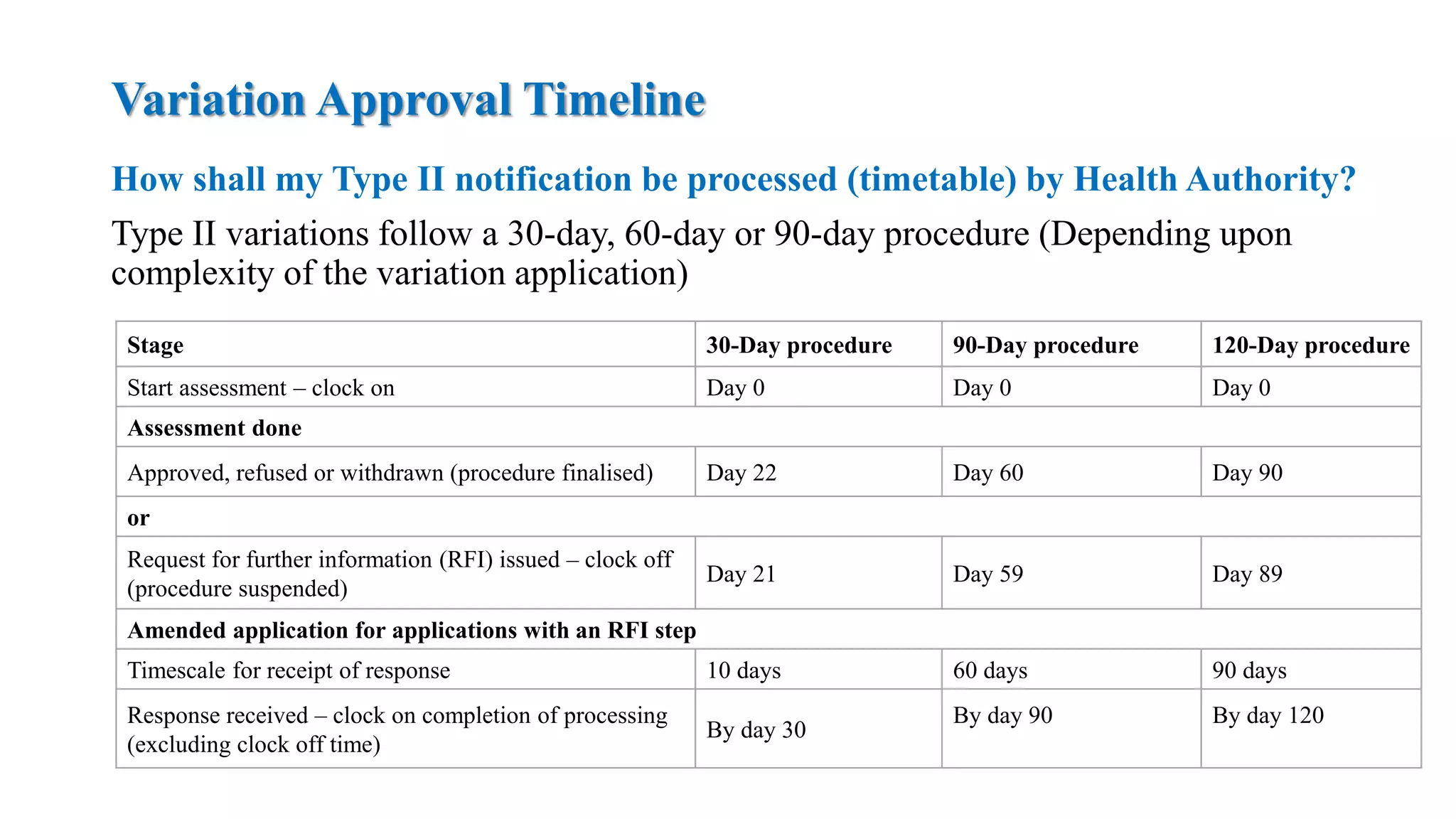
The document outlines the regulatory framework for variations in marketing authorizations for medicinal products in the EU, categorized into minor (Type-IA, Type-IB) and major (Type-II) variations. It details submission procedures, timelines, examples of variations, and fees, emphasizing the implications for product quality, safety, and efficacy. Additionally, it covers renewal processes for marketing authorizations and provides guidance on documentation and grouping of variations.