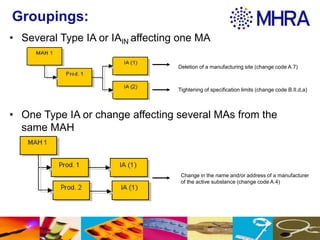
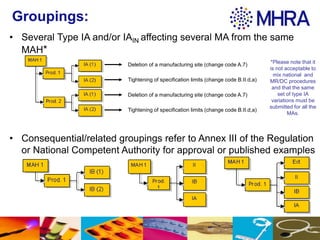
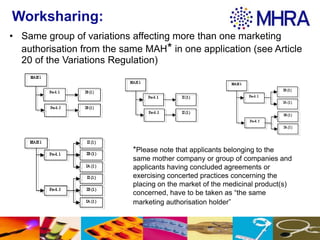
This document outlines different groupings for type IA variations that can be submitted together, including several type IA variations affecting one or multiple marketing authorizations from the same marketing authorization holder. It also allows for related groupings defined in Annex III of the Regulation or by national competent authorities. The document provides examples of type IA variations that can be grouped, such as deletion of a manufacturing site or tightening of specification limits. It describes worksharing where the same group of variations can affect multiple marketing authorizations from the same marketing authorization holder in a single application.