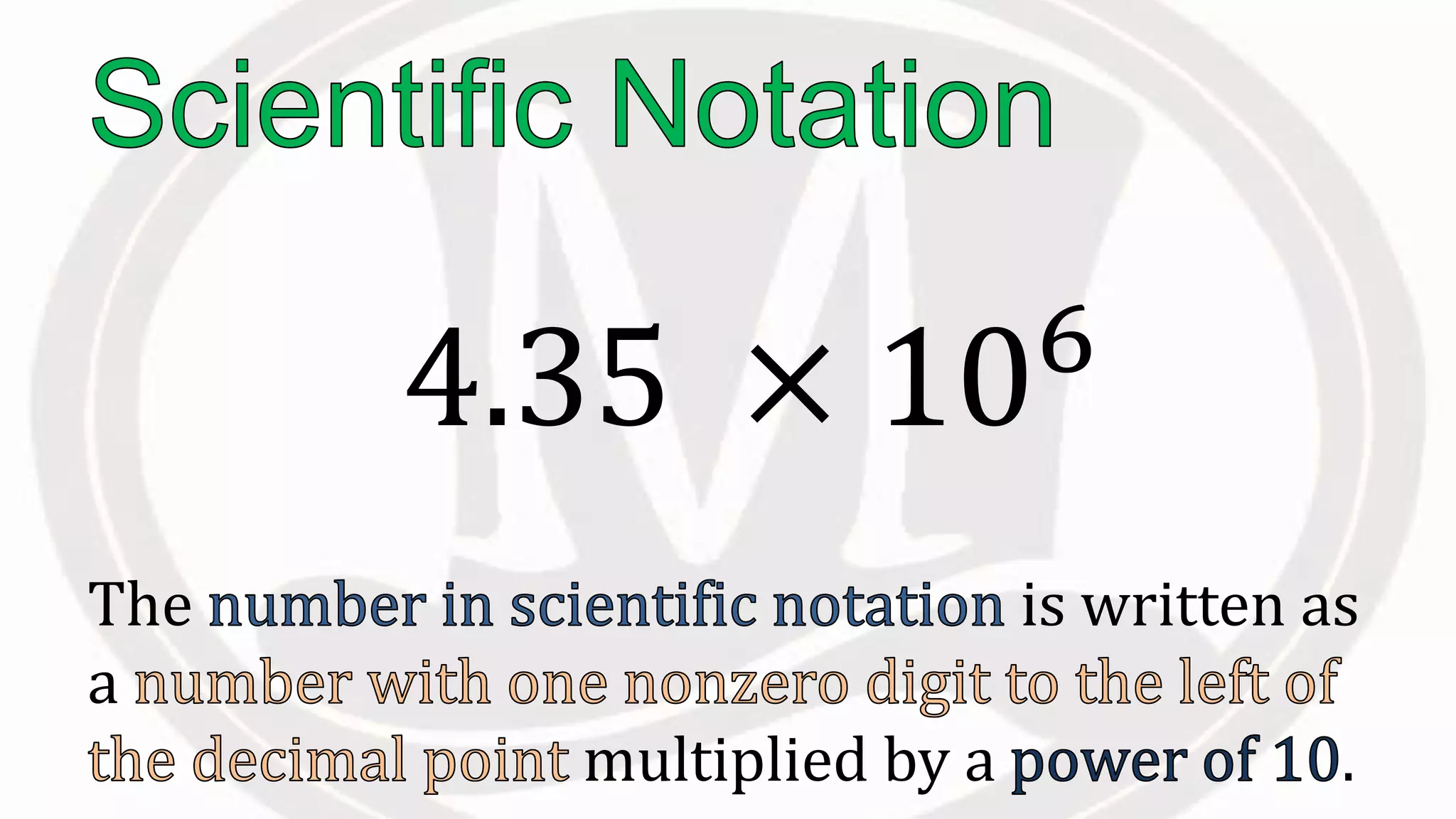
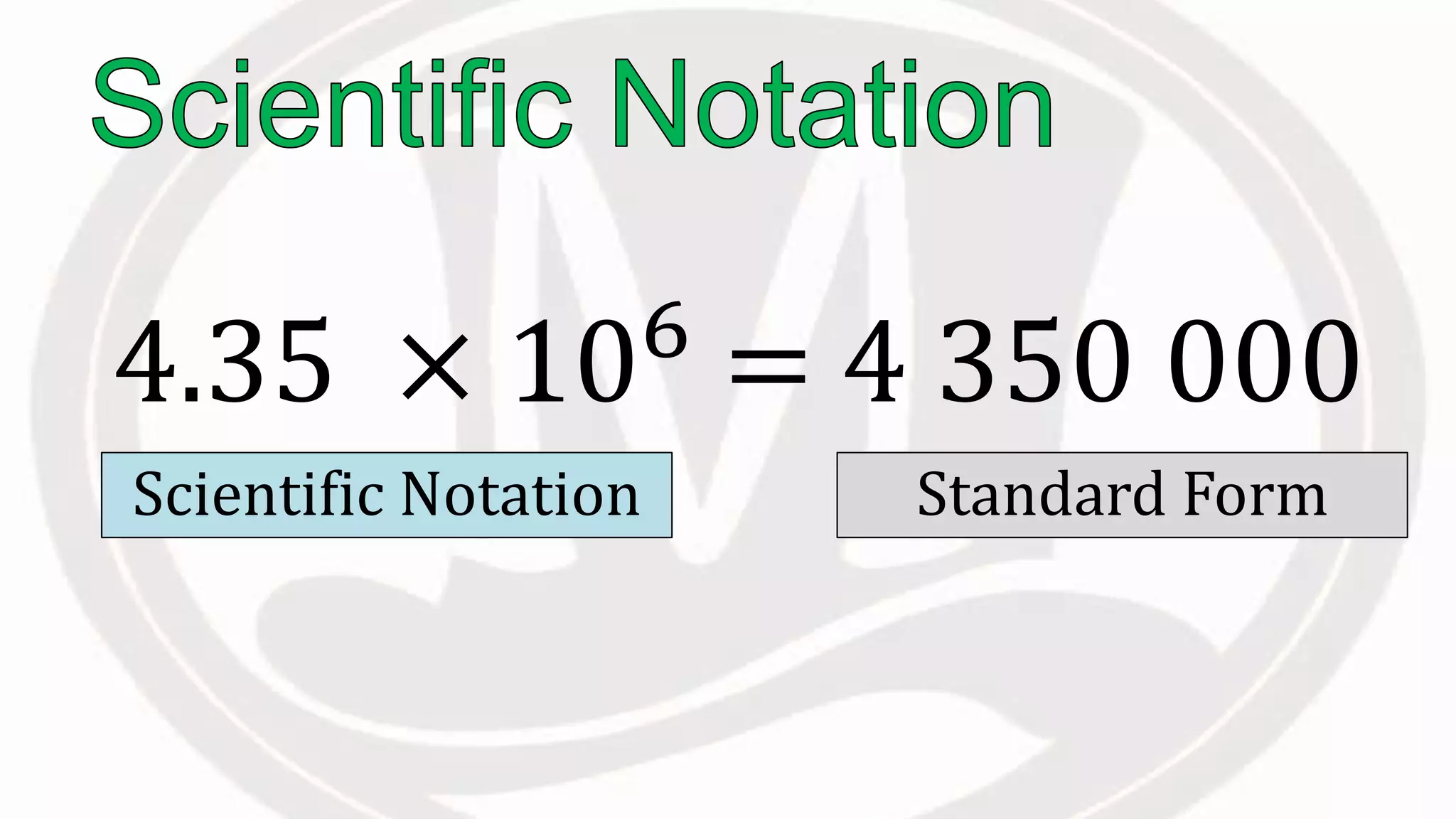
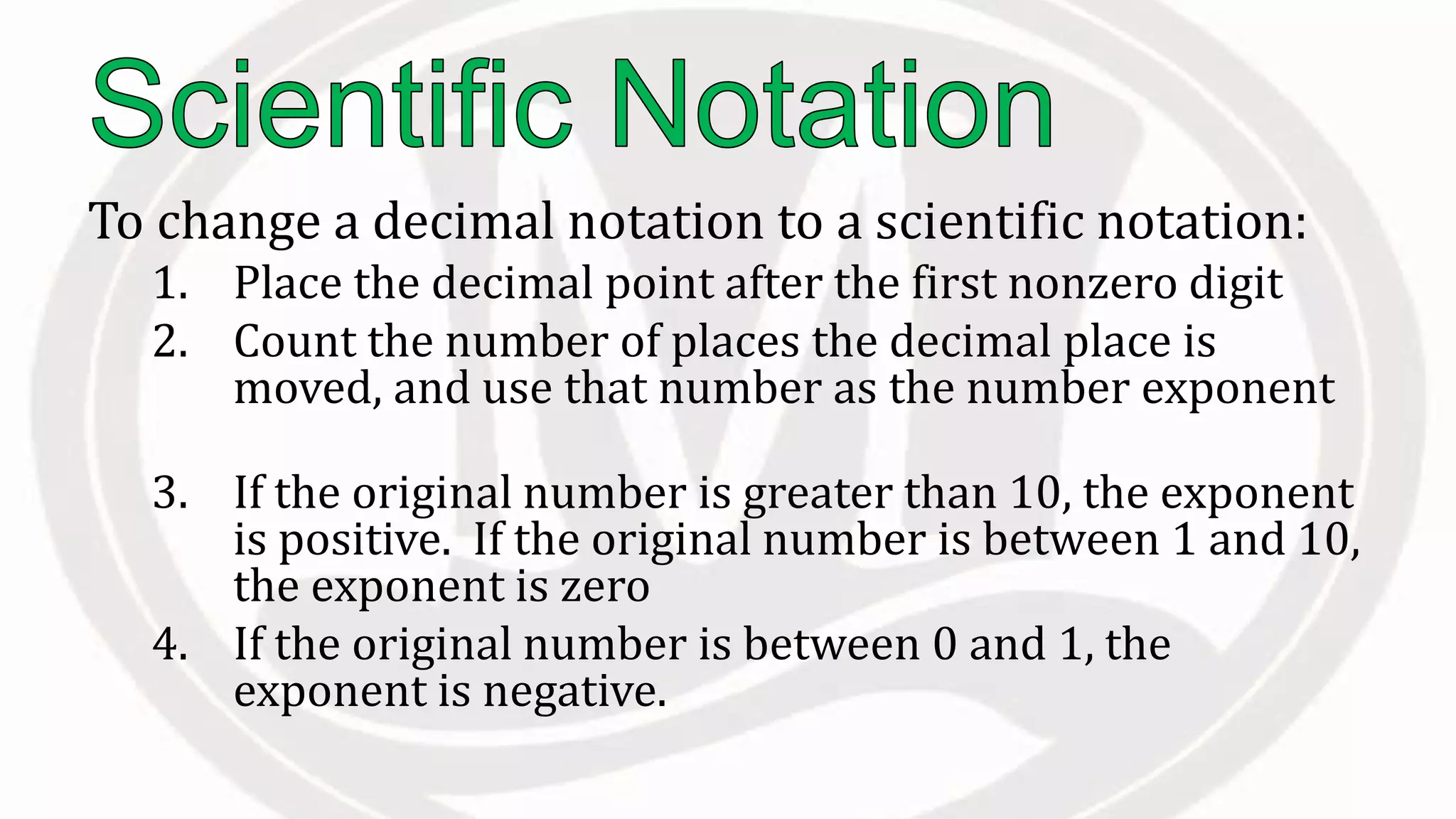
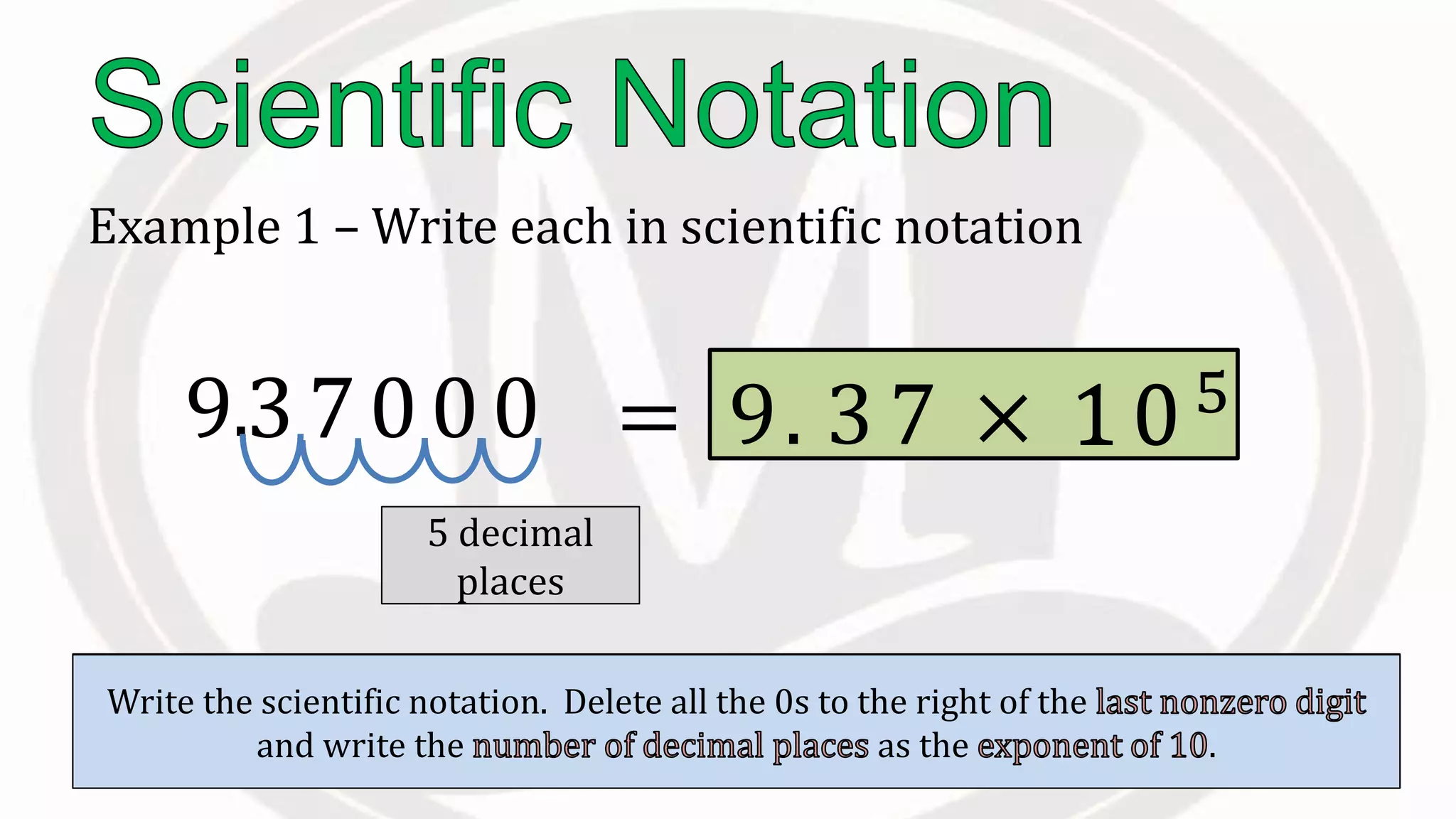
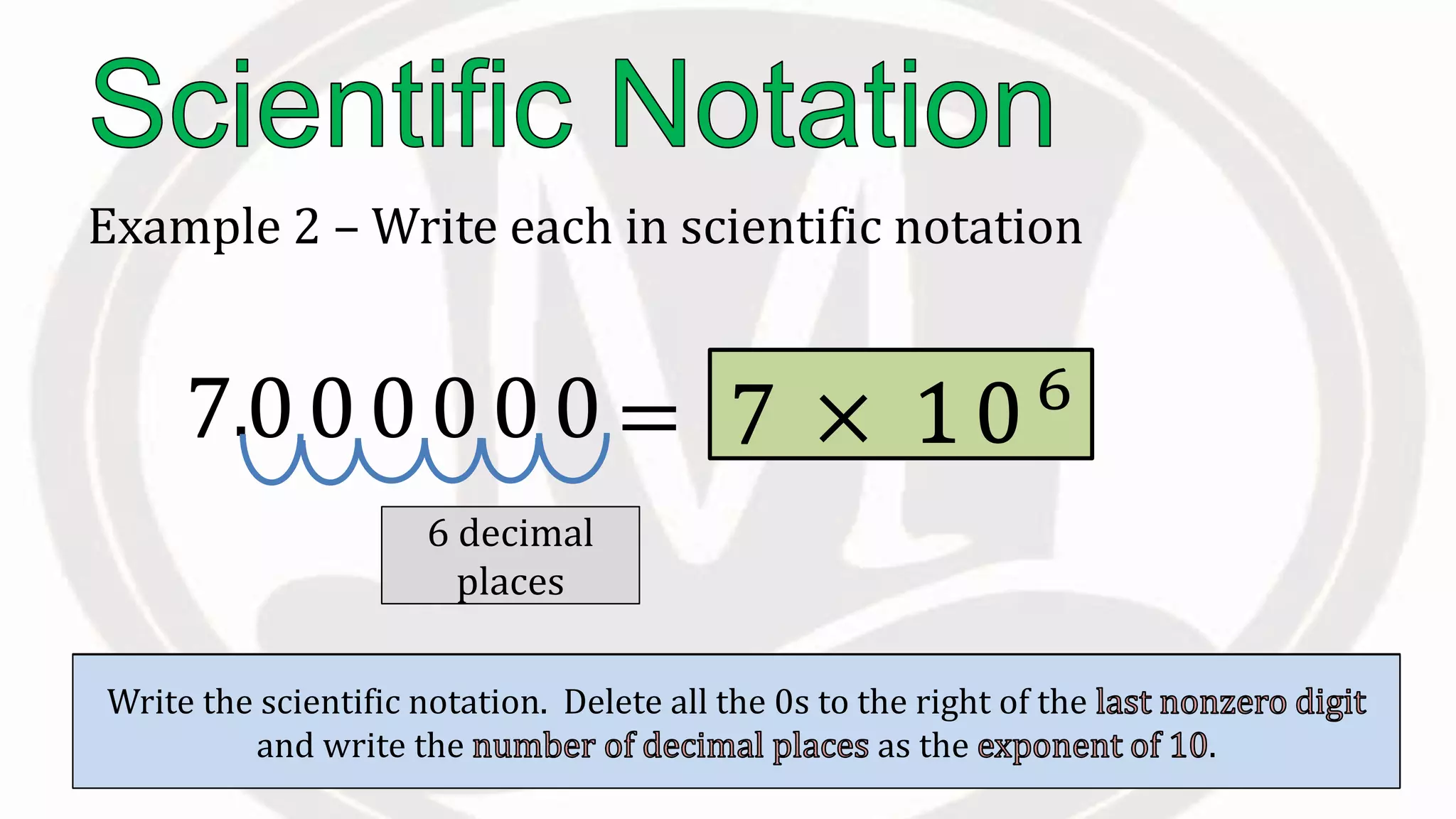
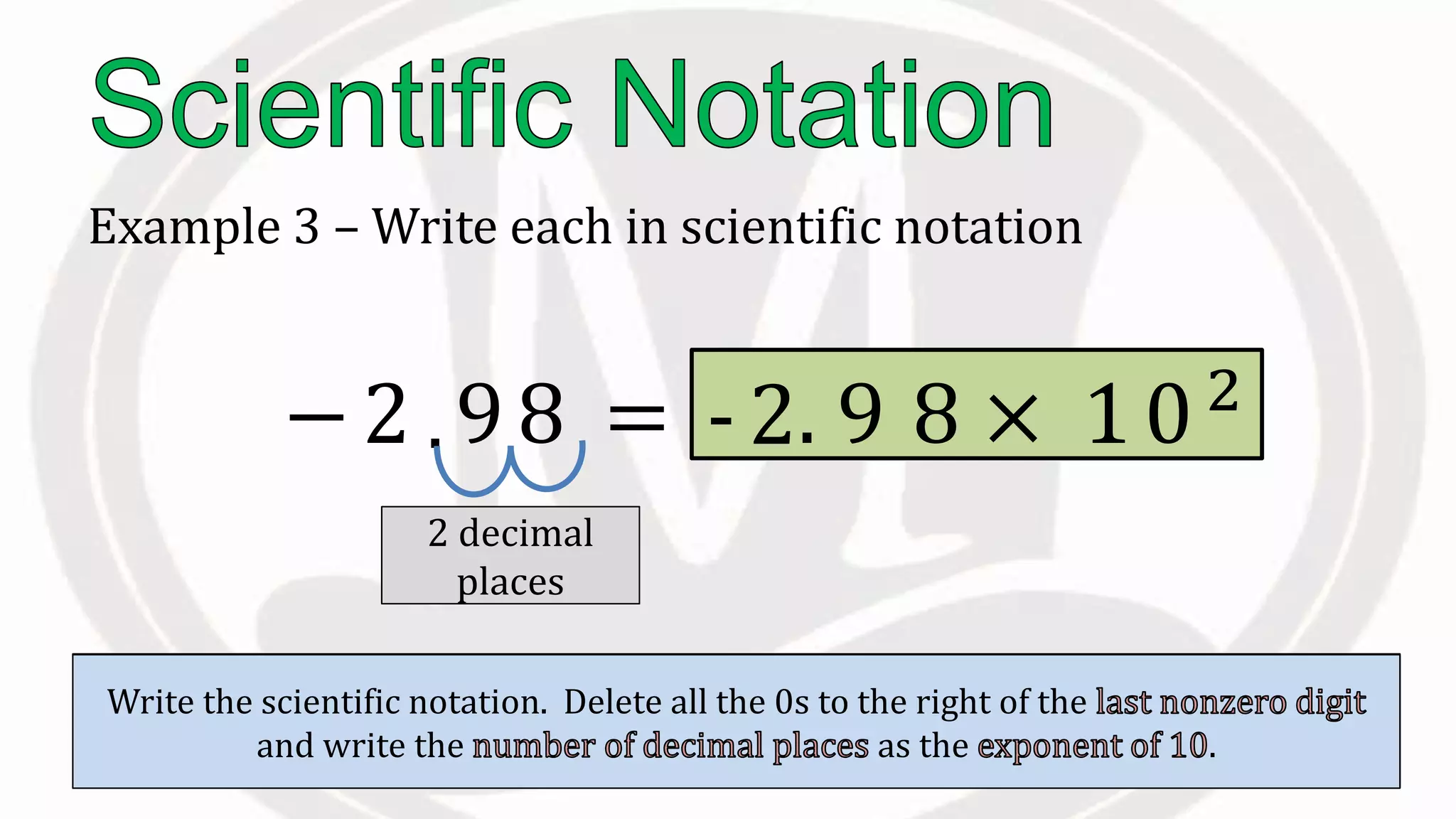
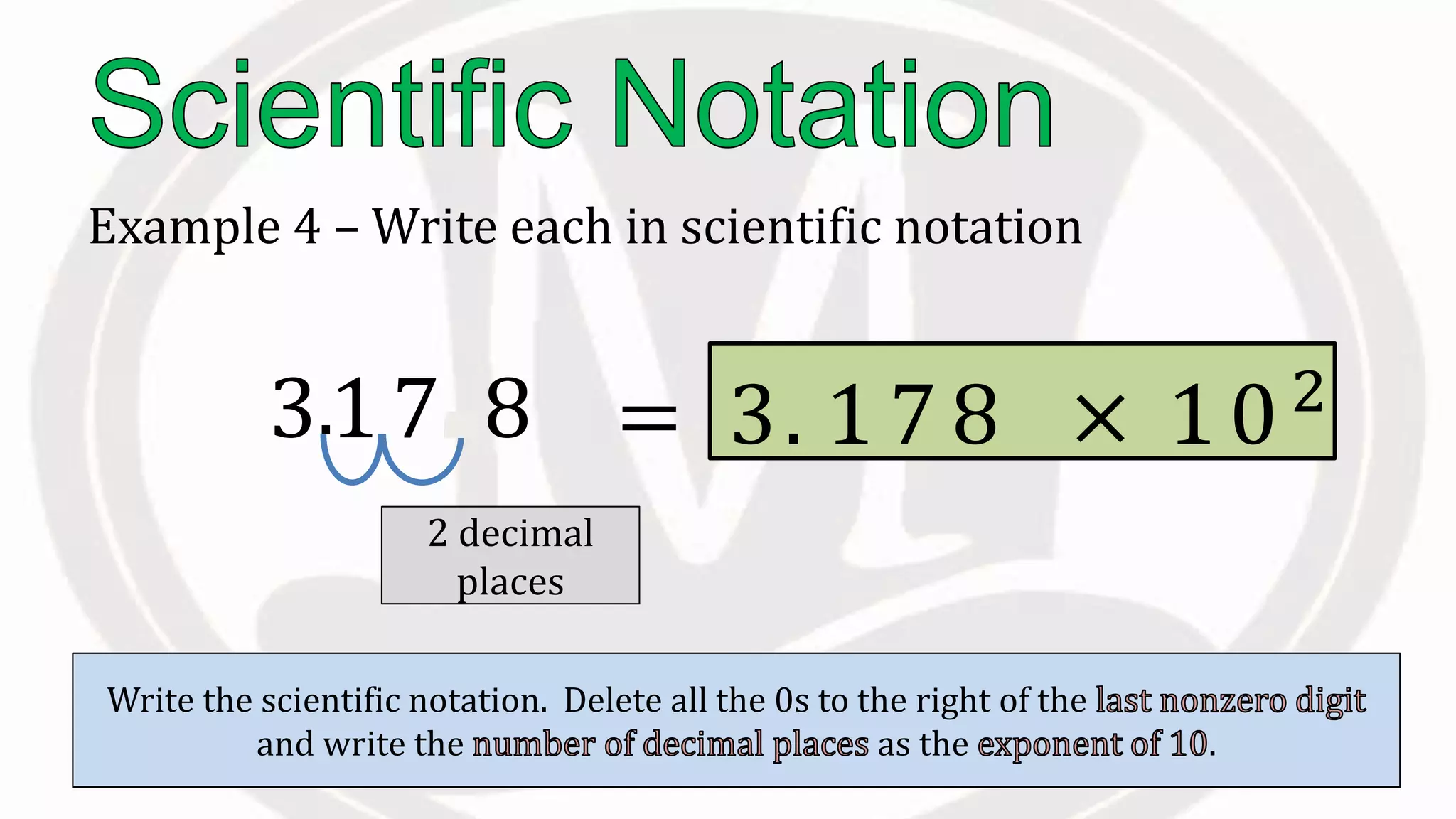
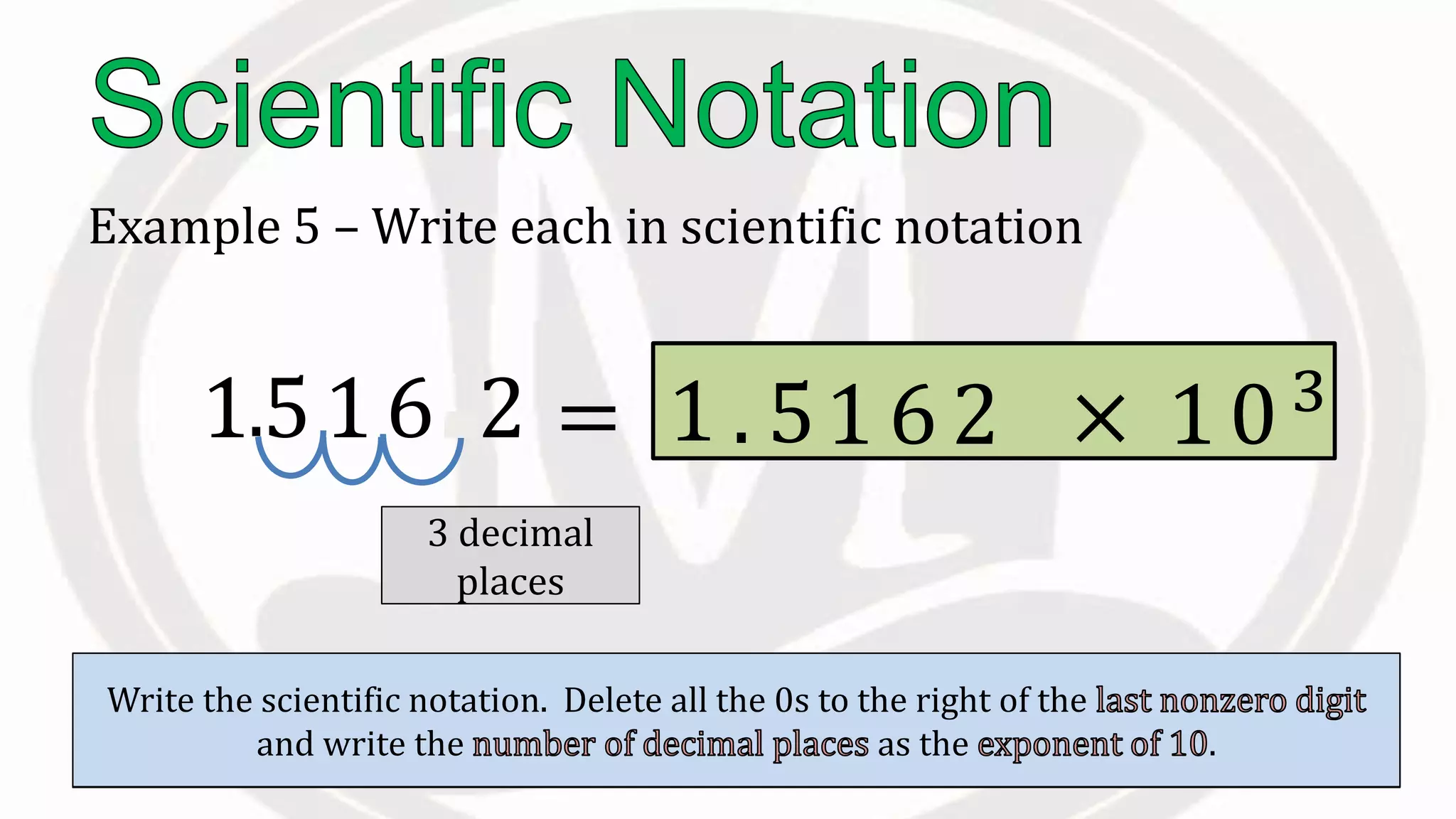
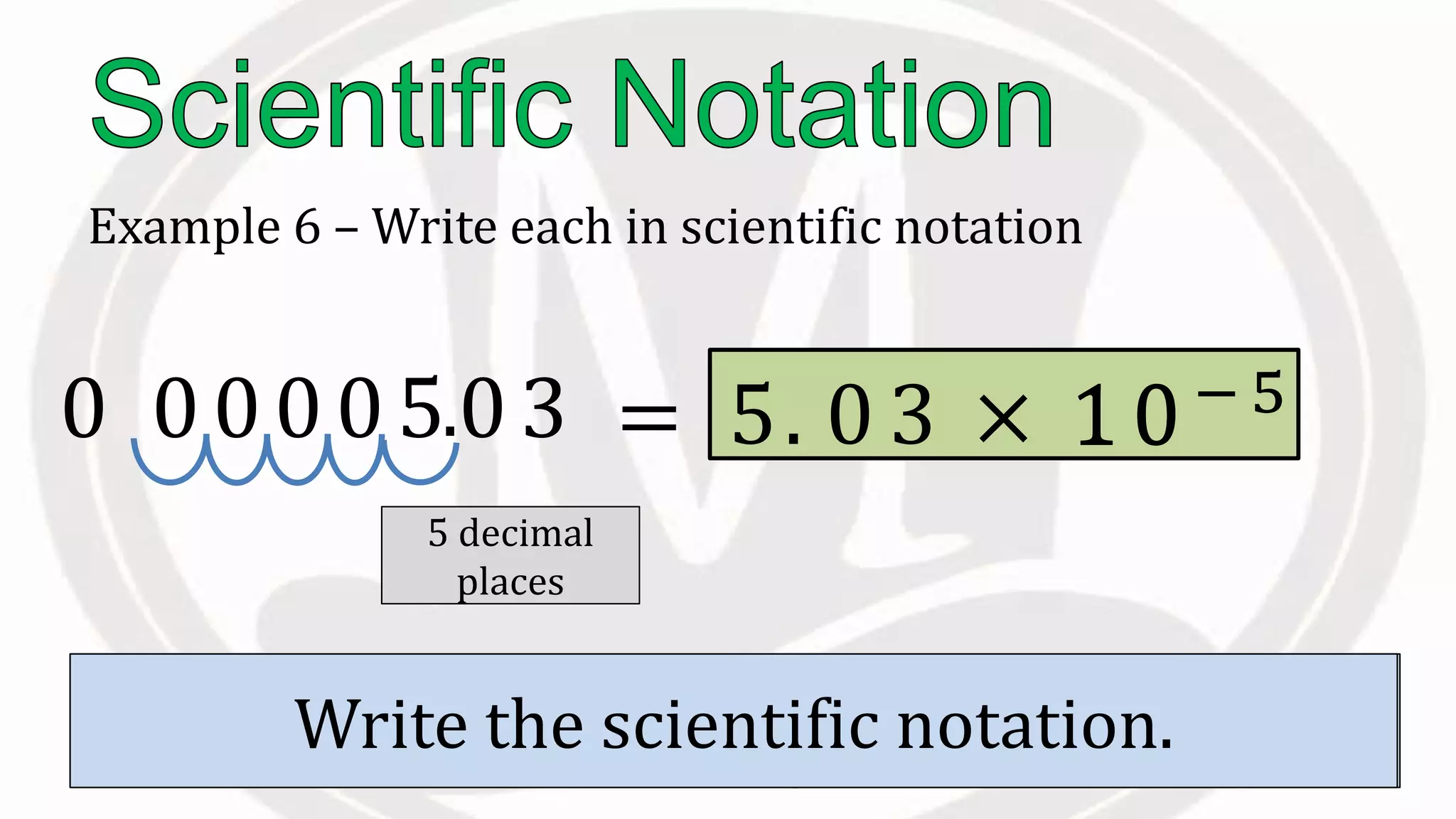
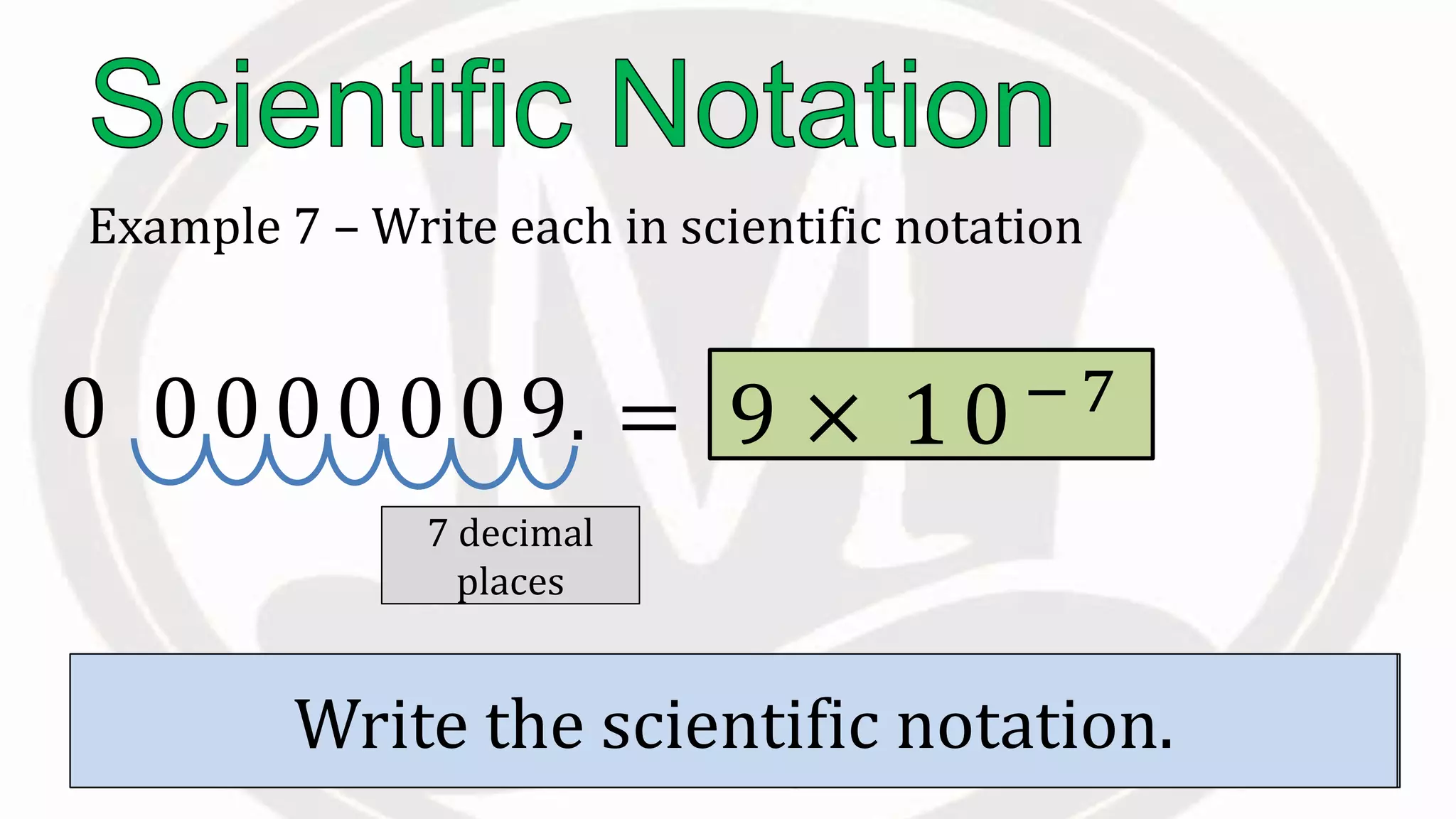
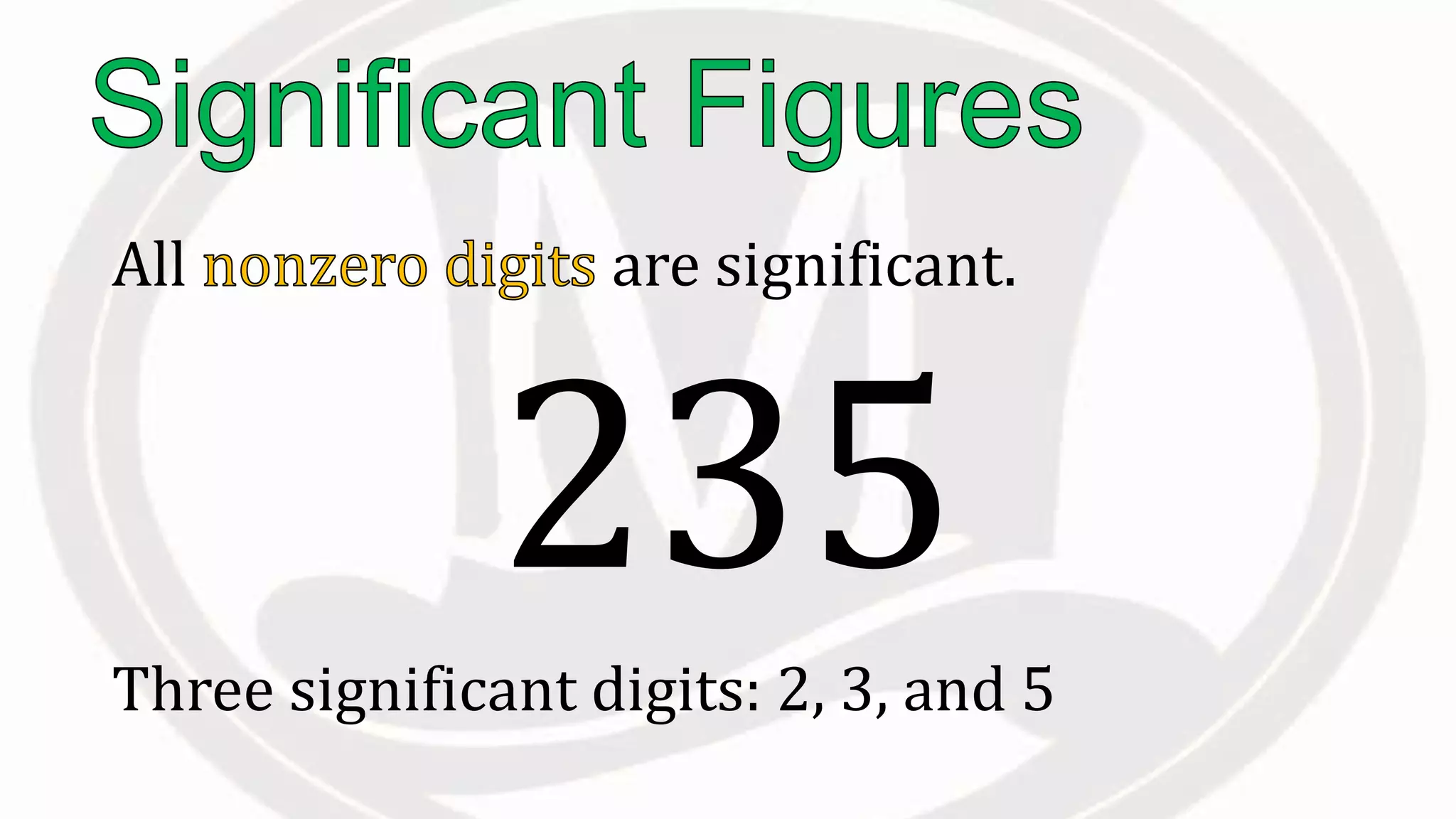
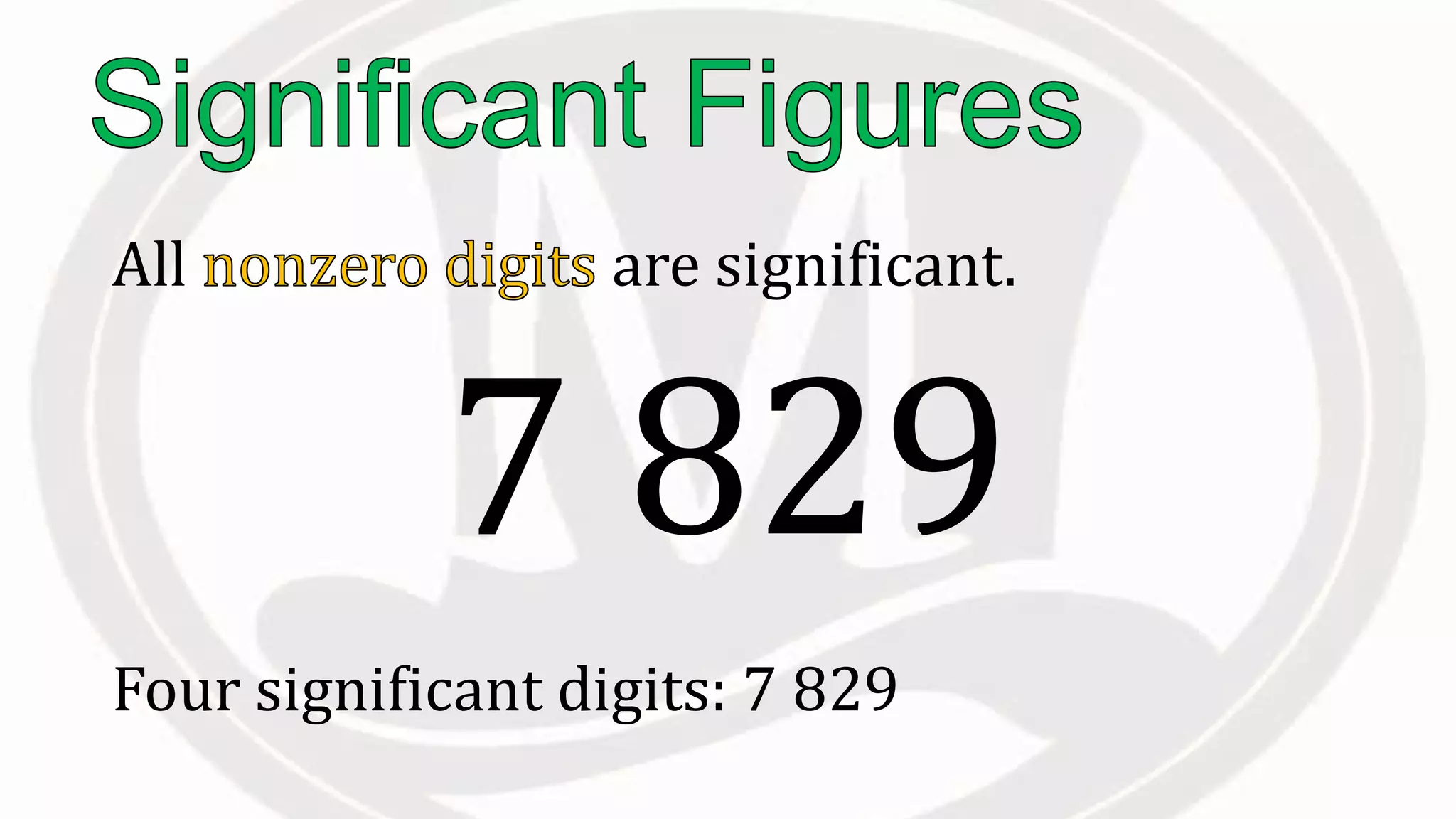
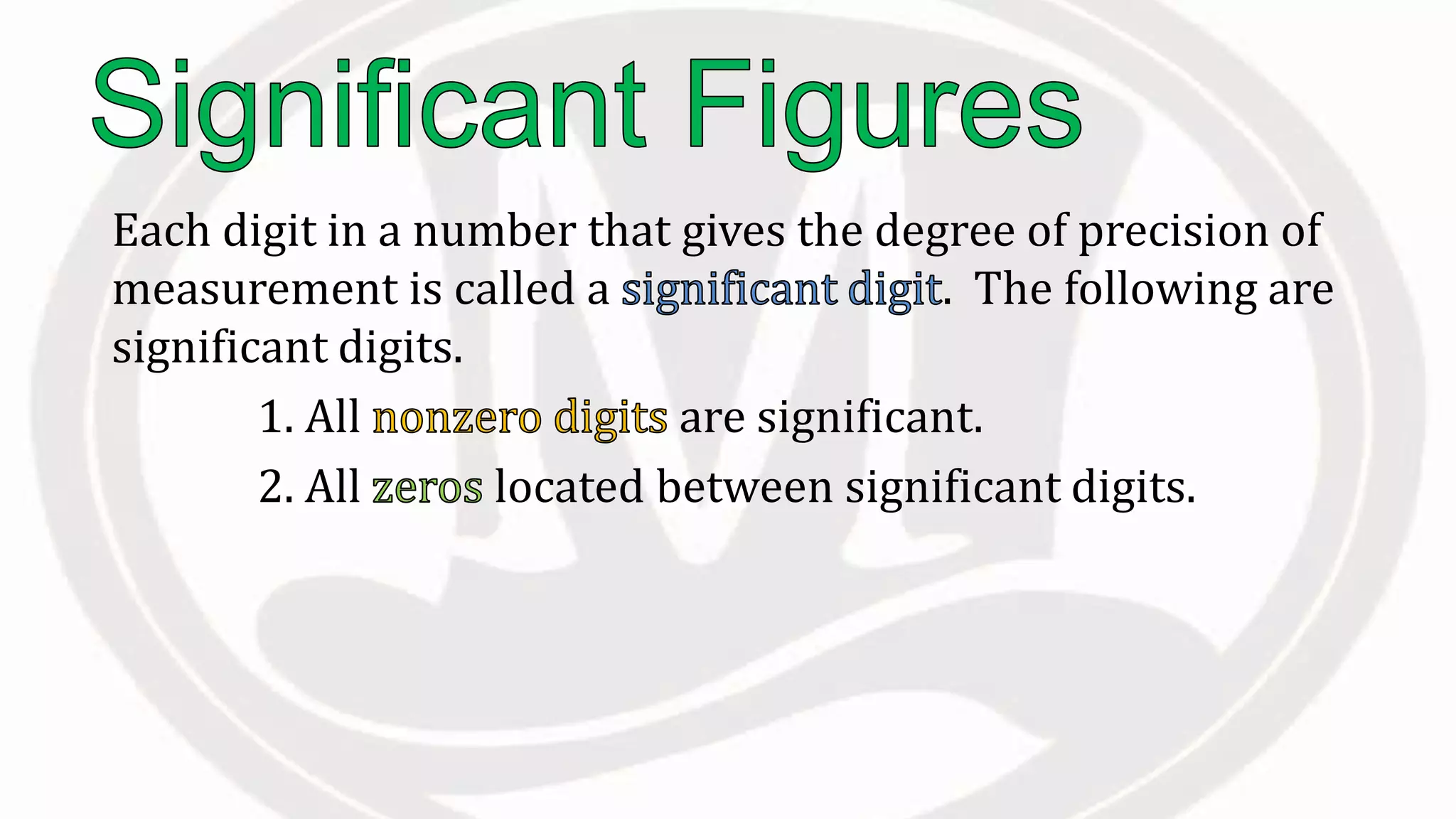
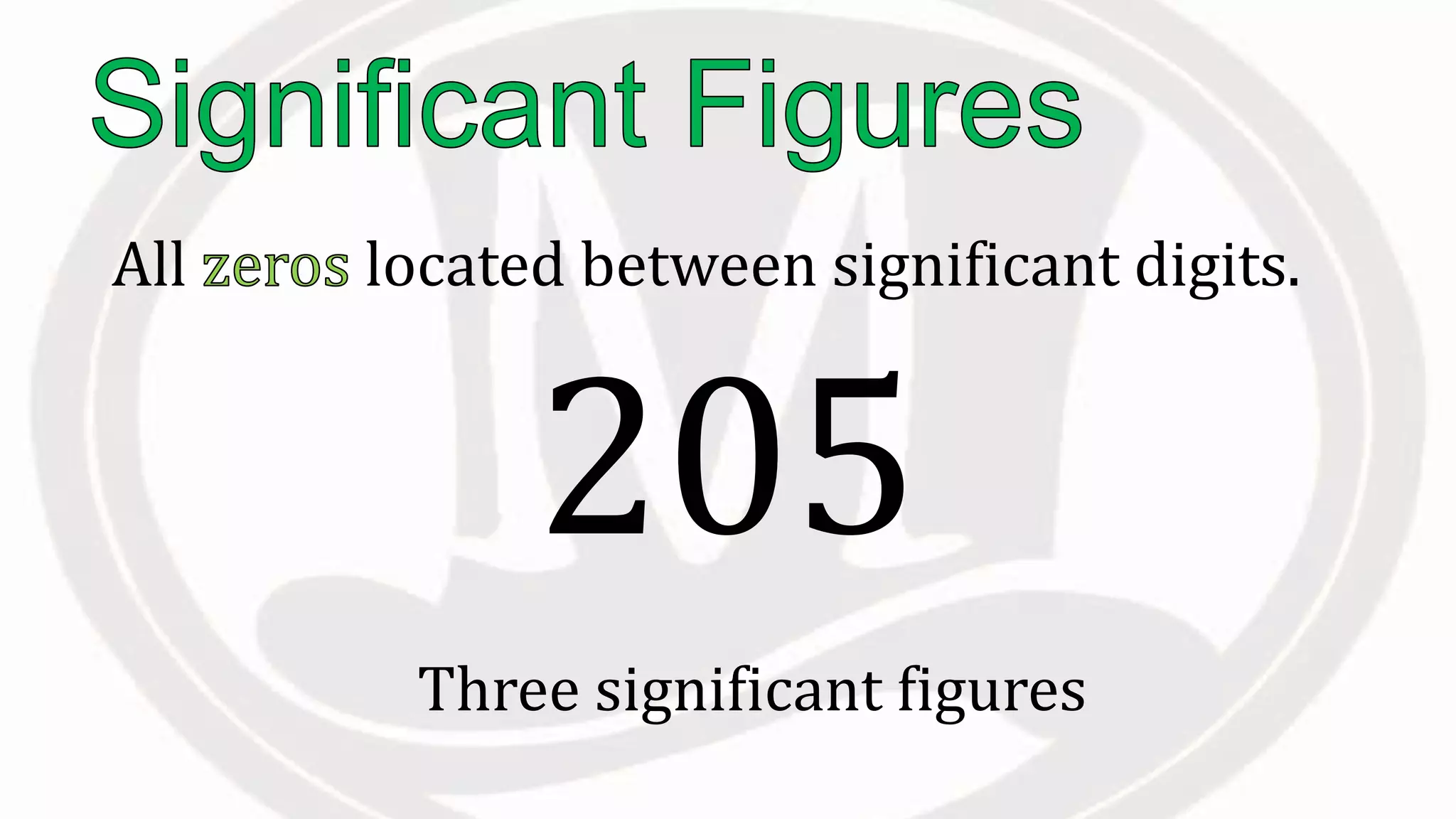
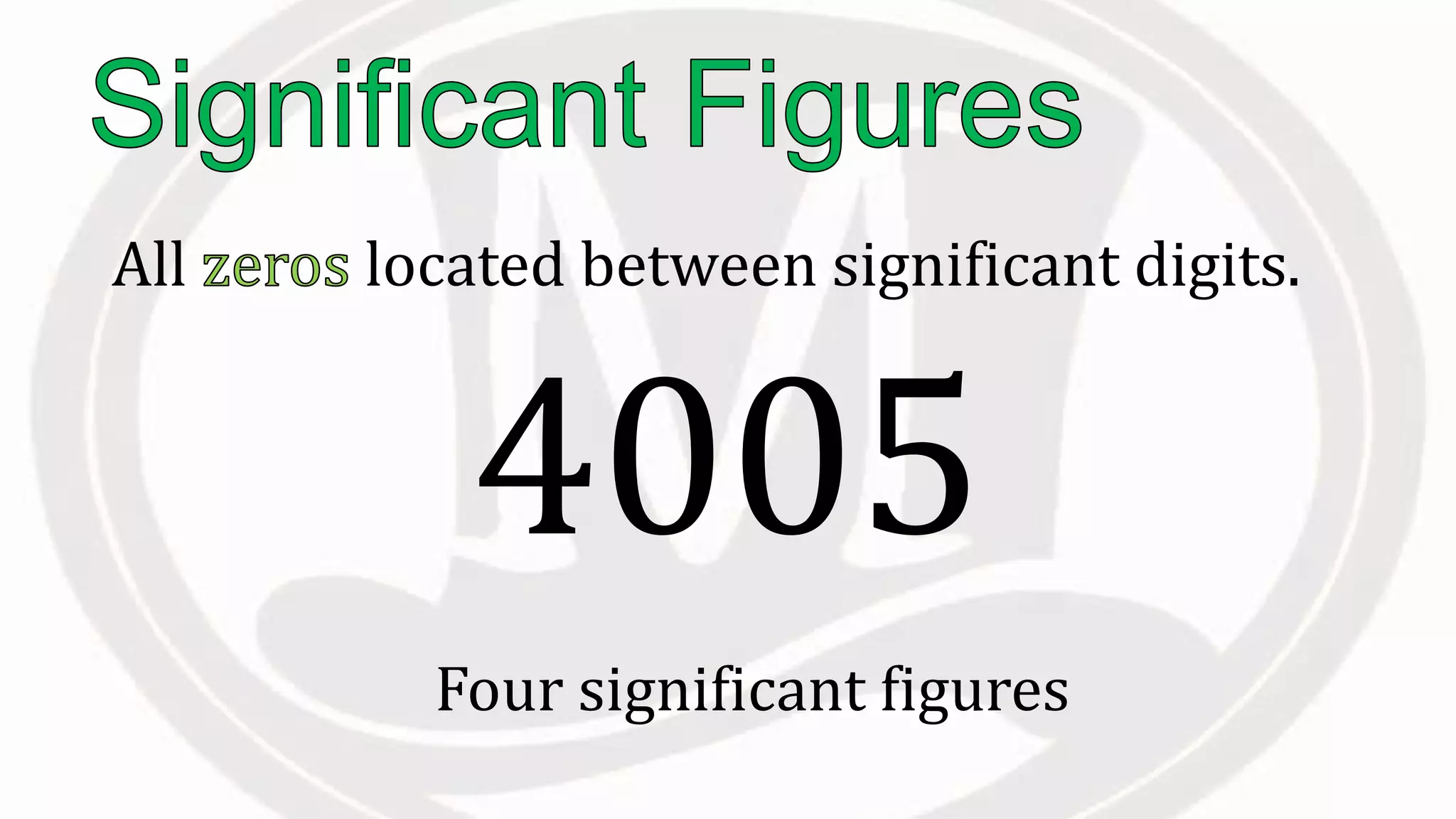
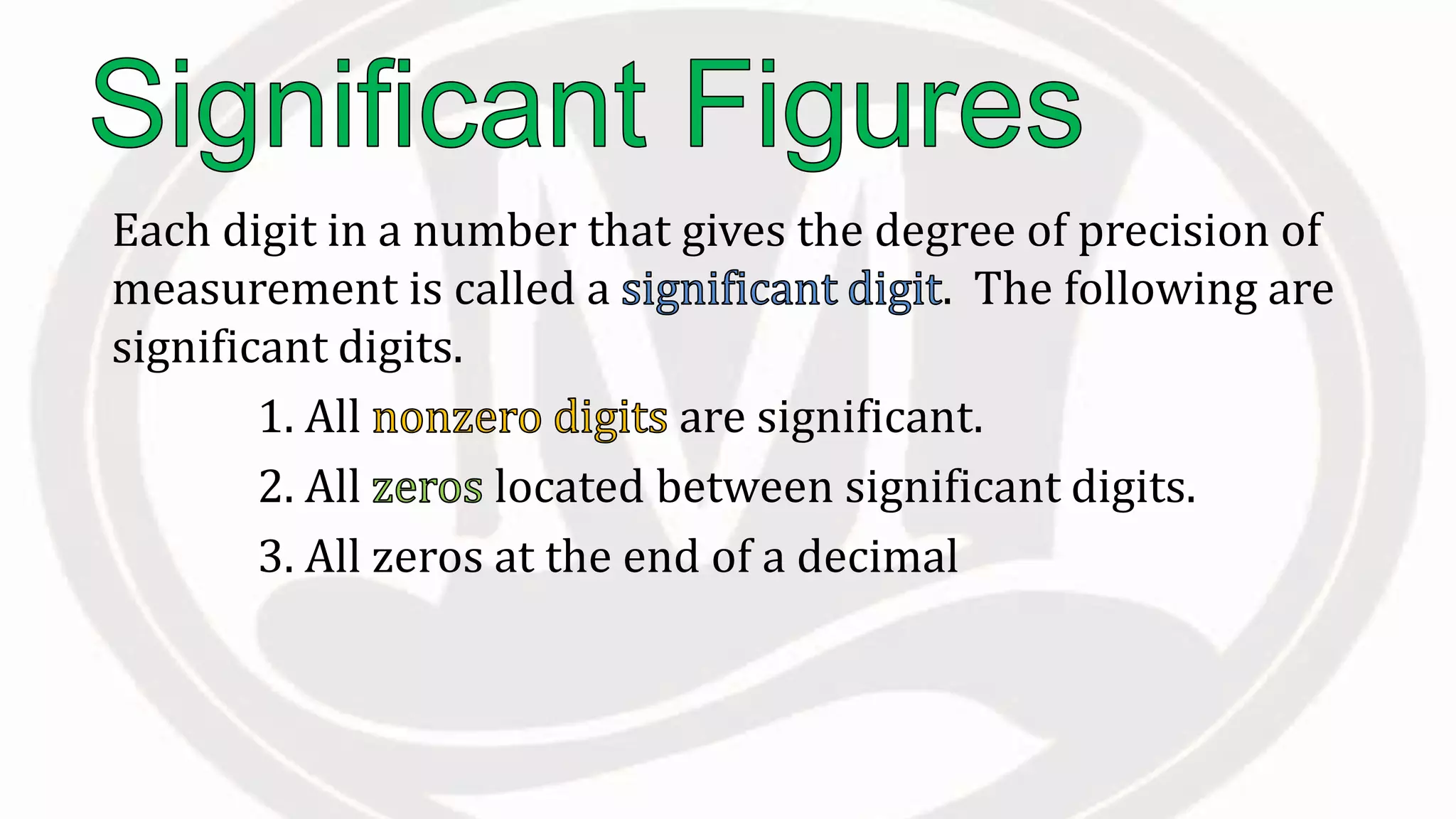
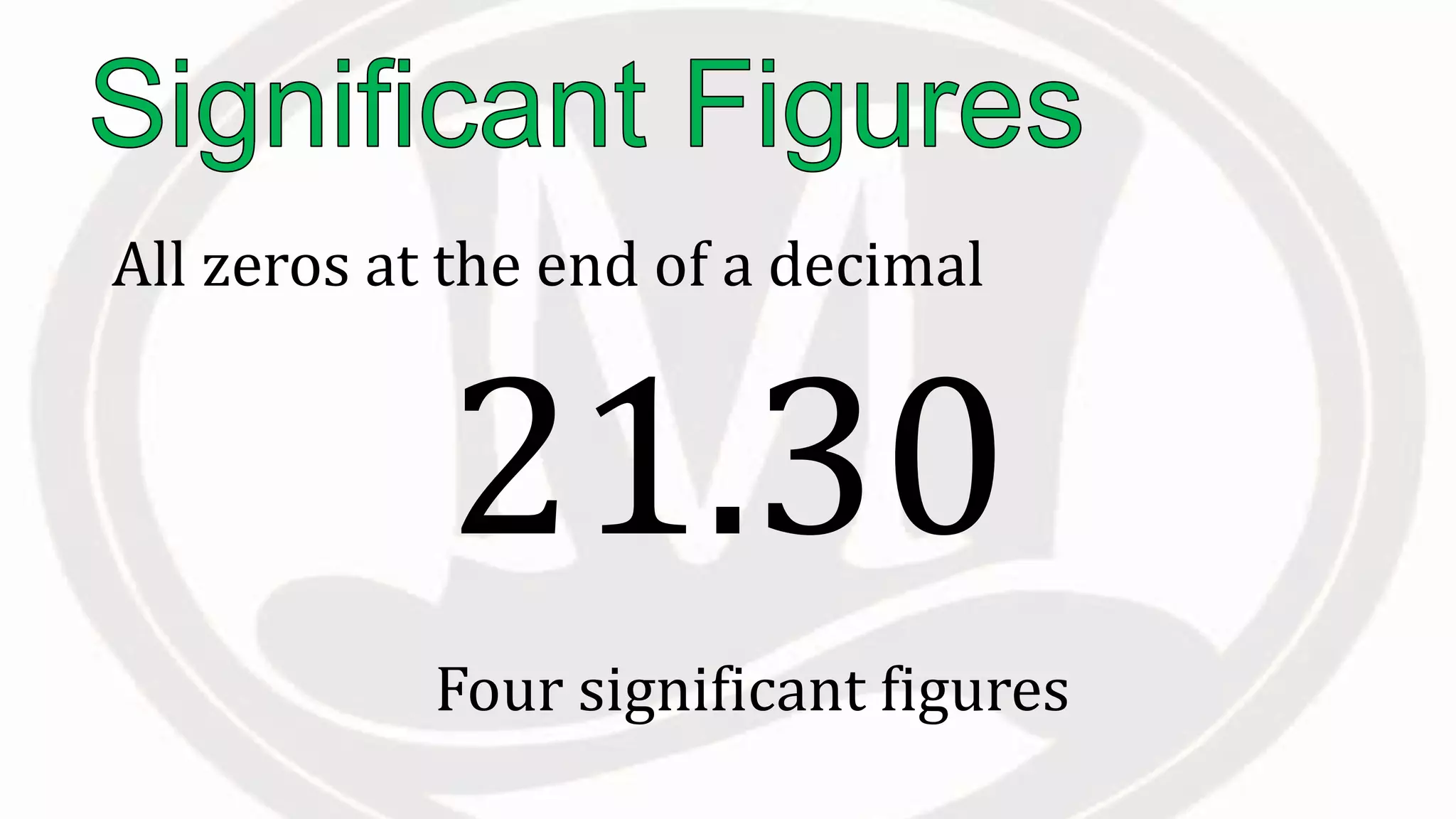
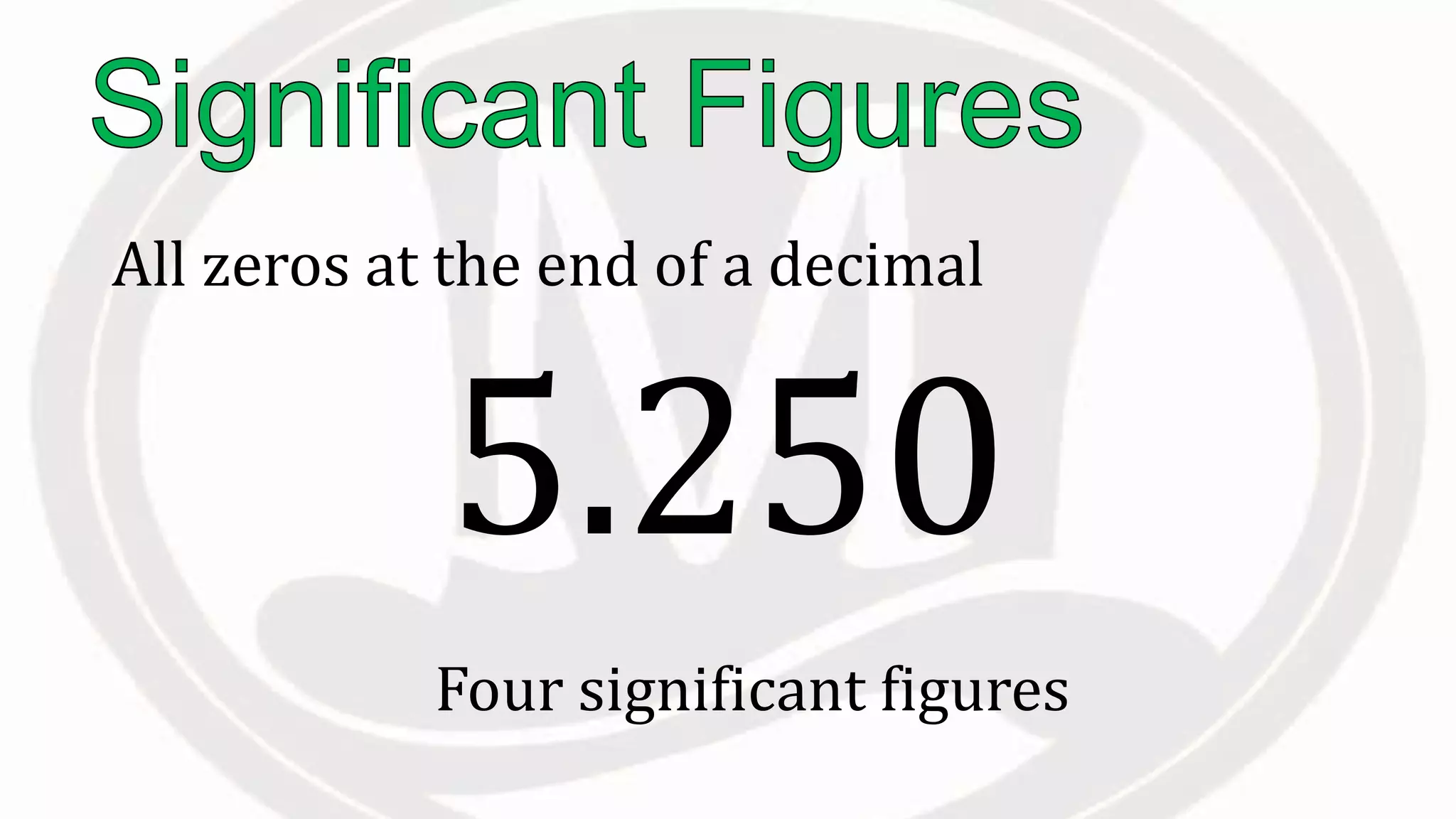
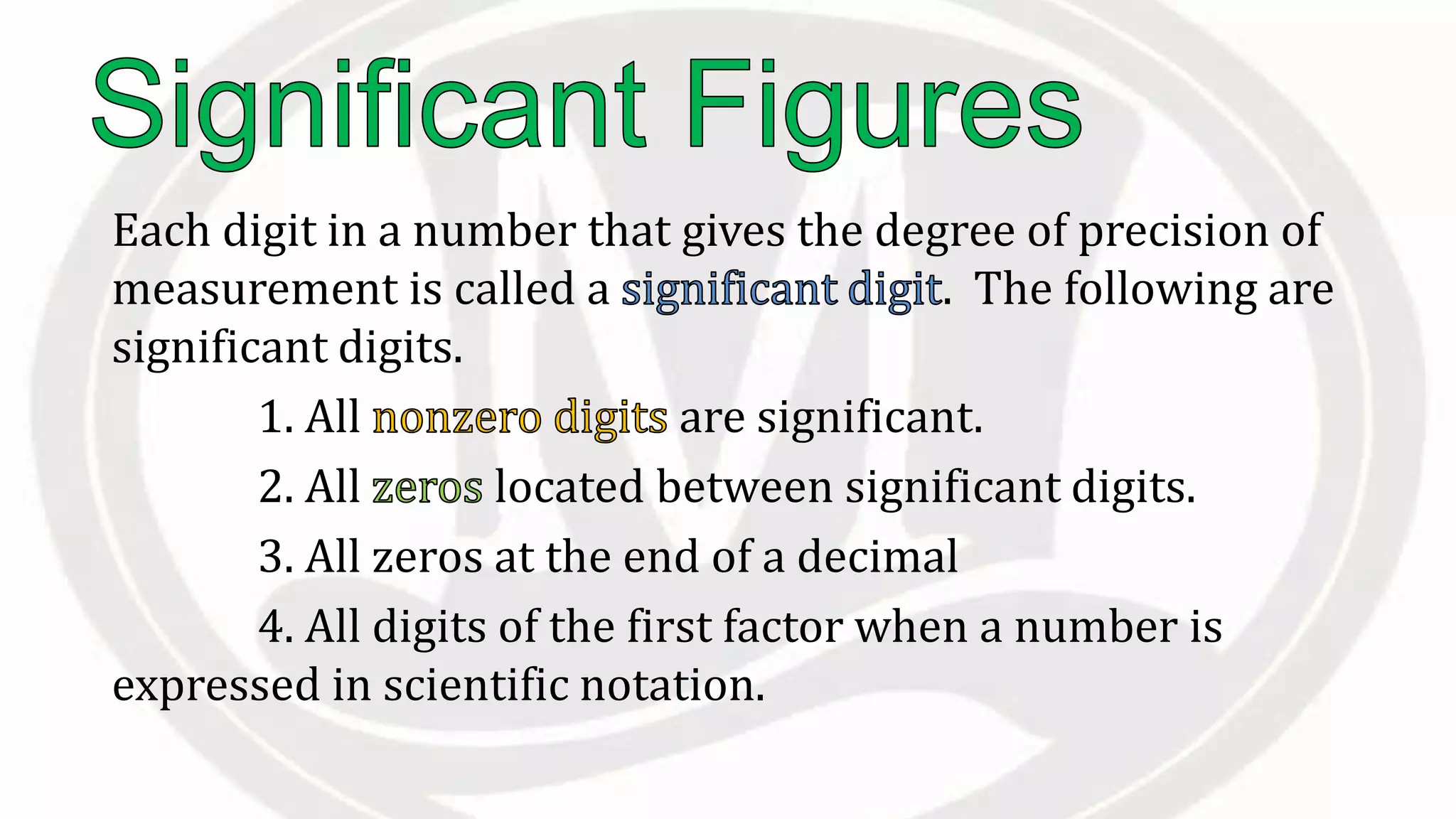
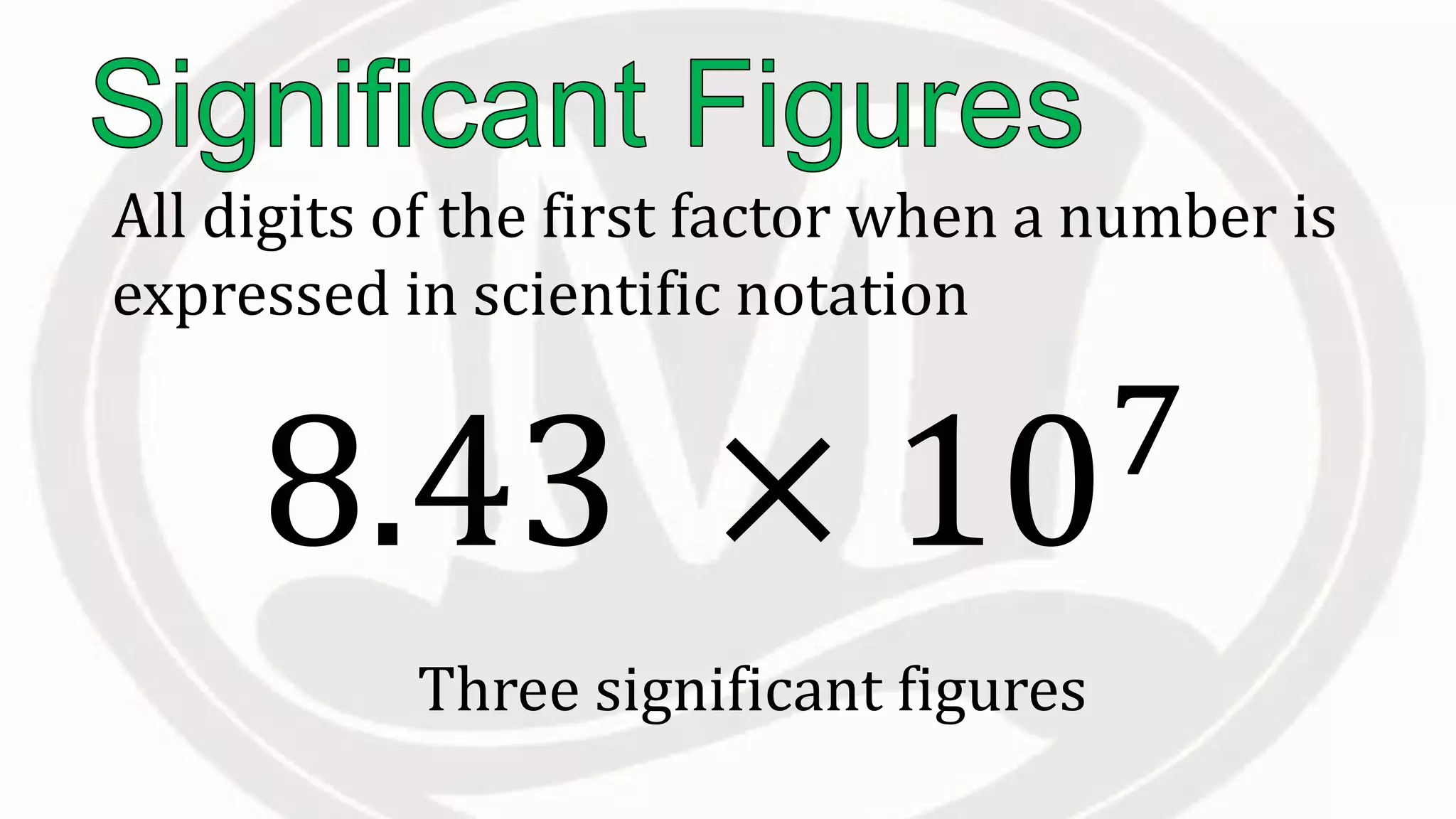
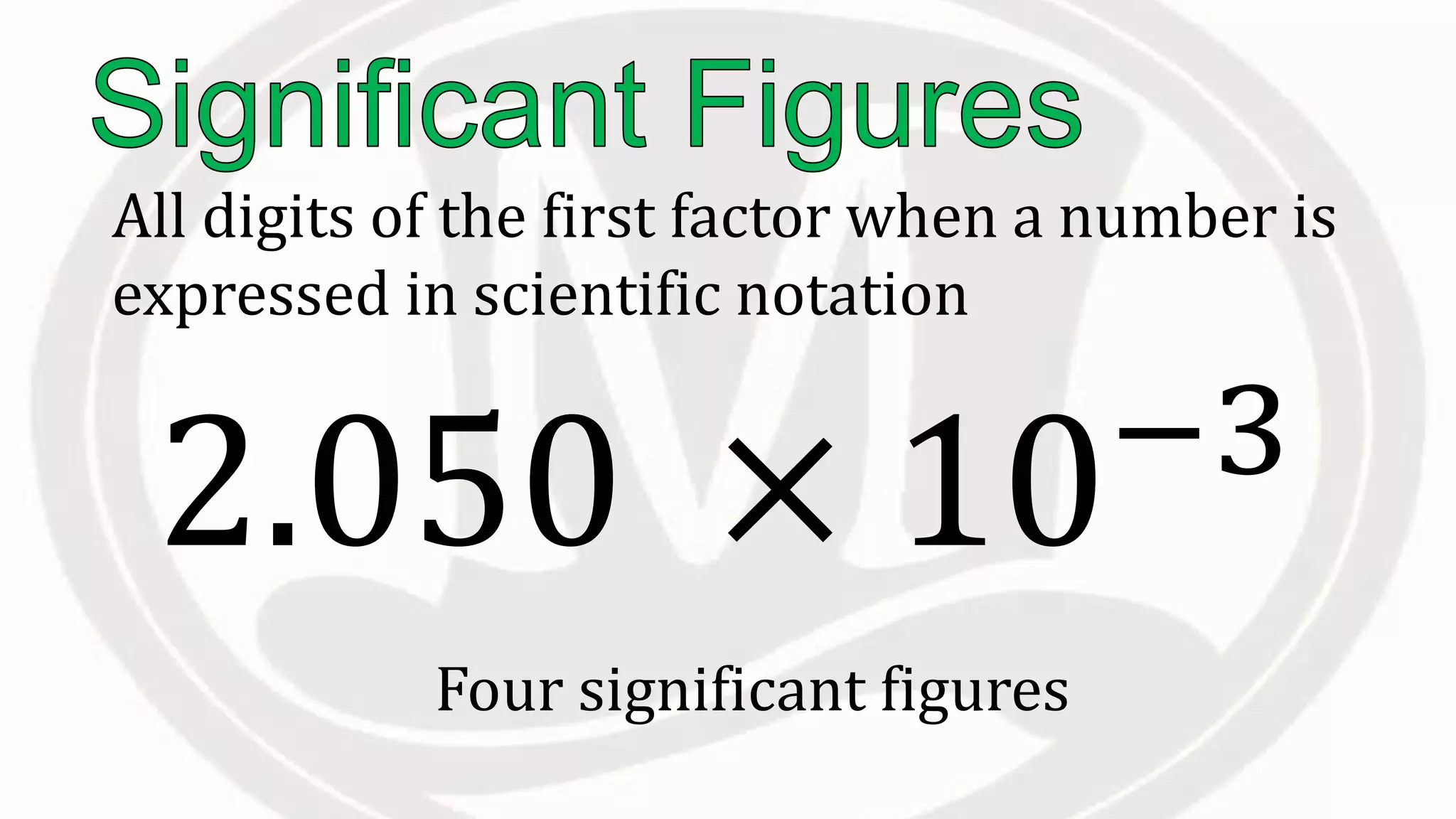
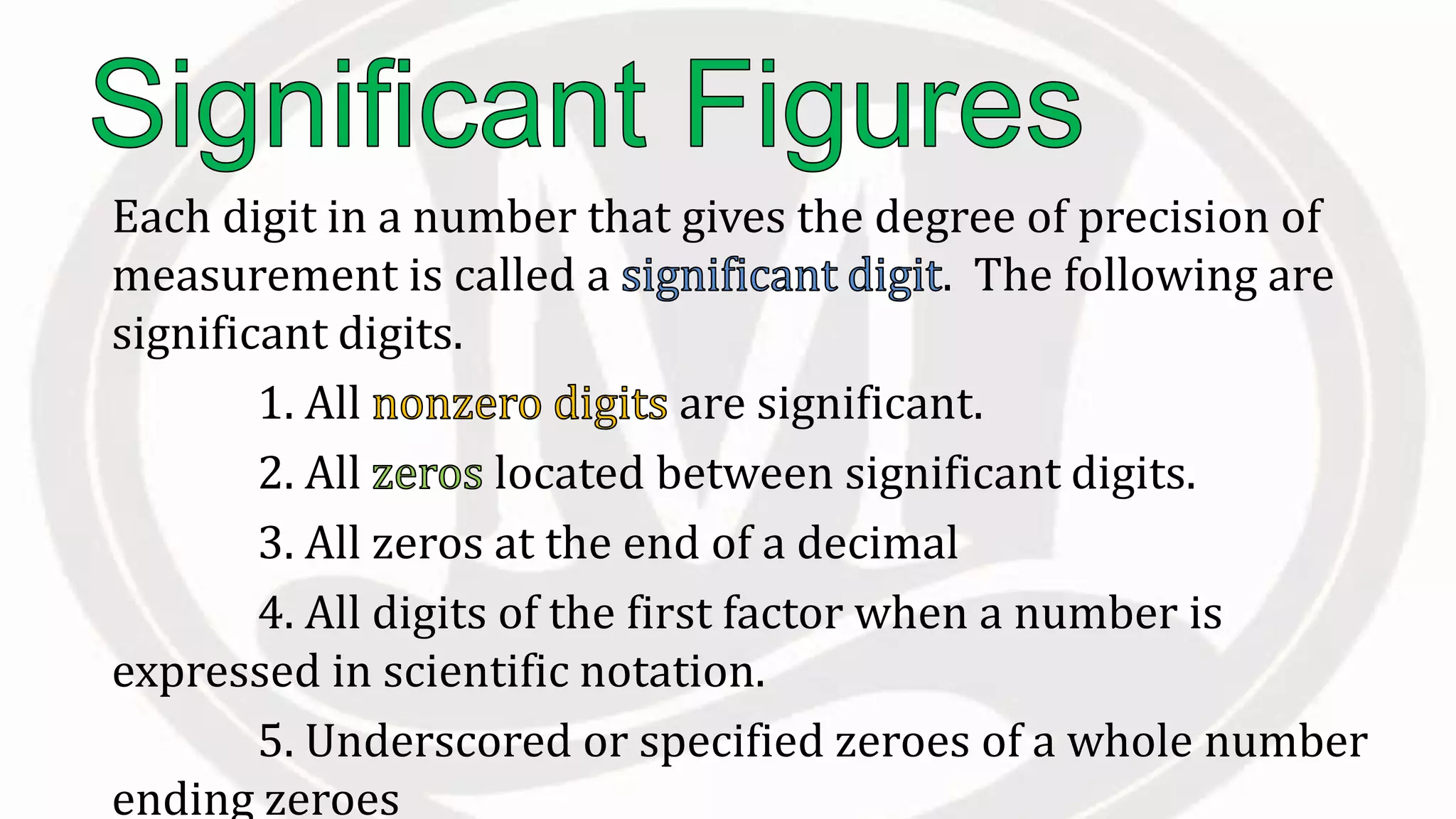
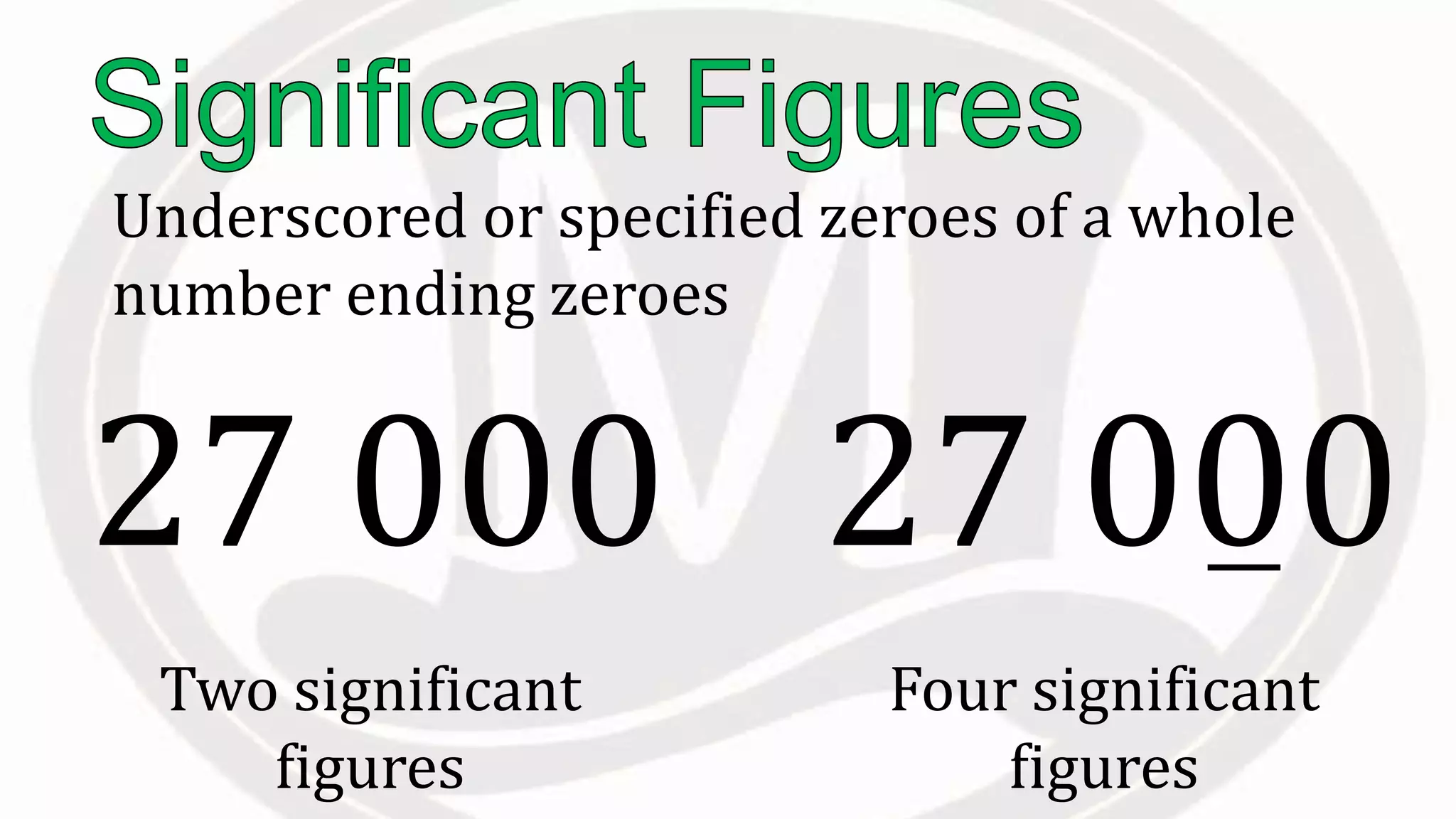
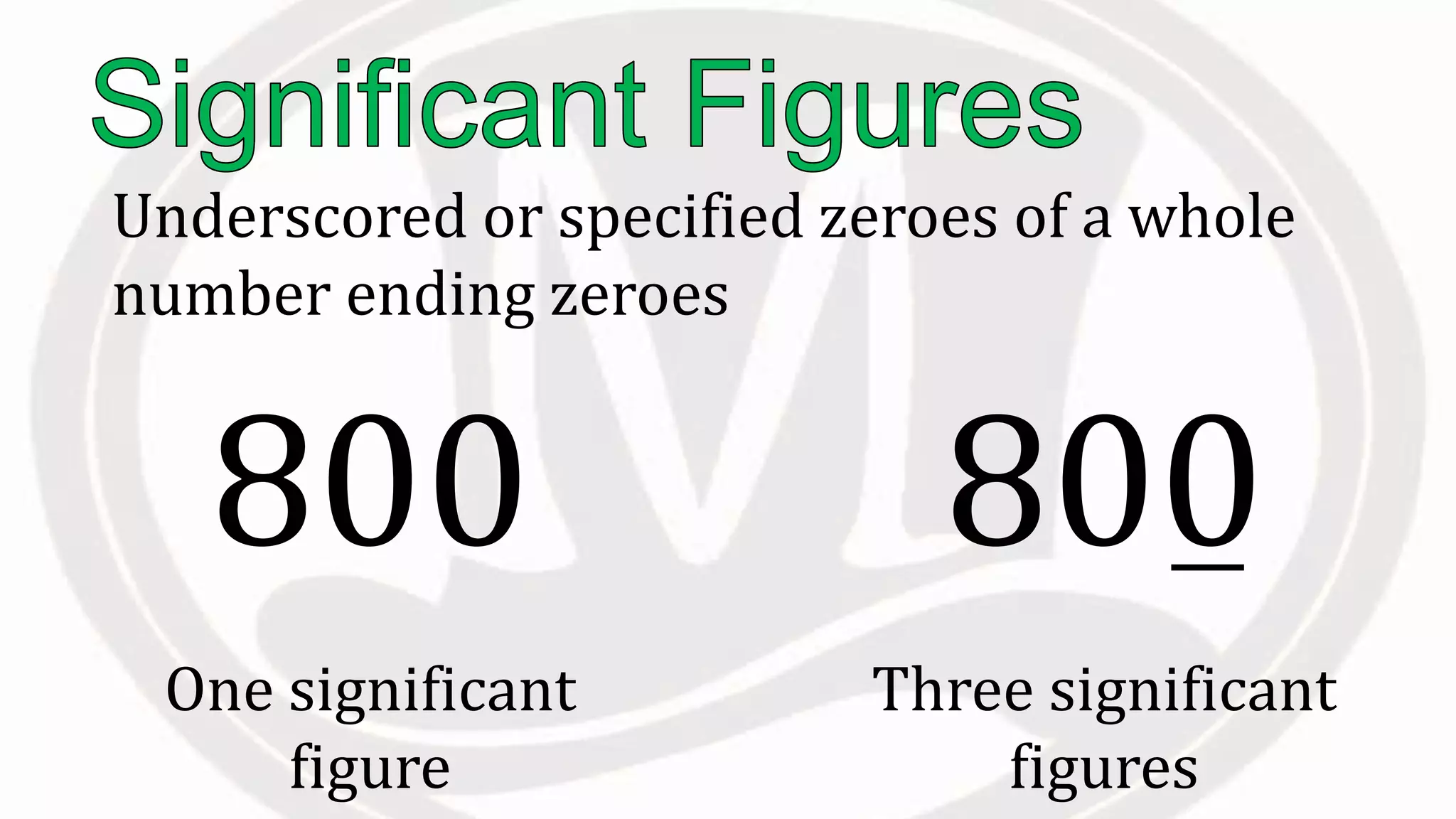
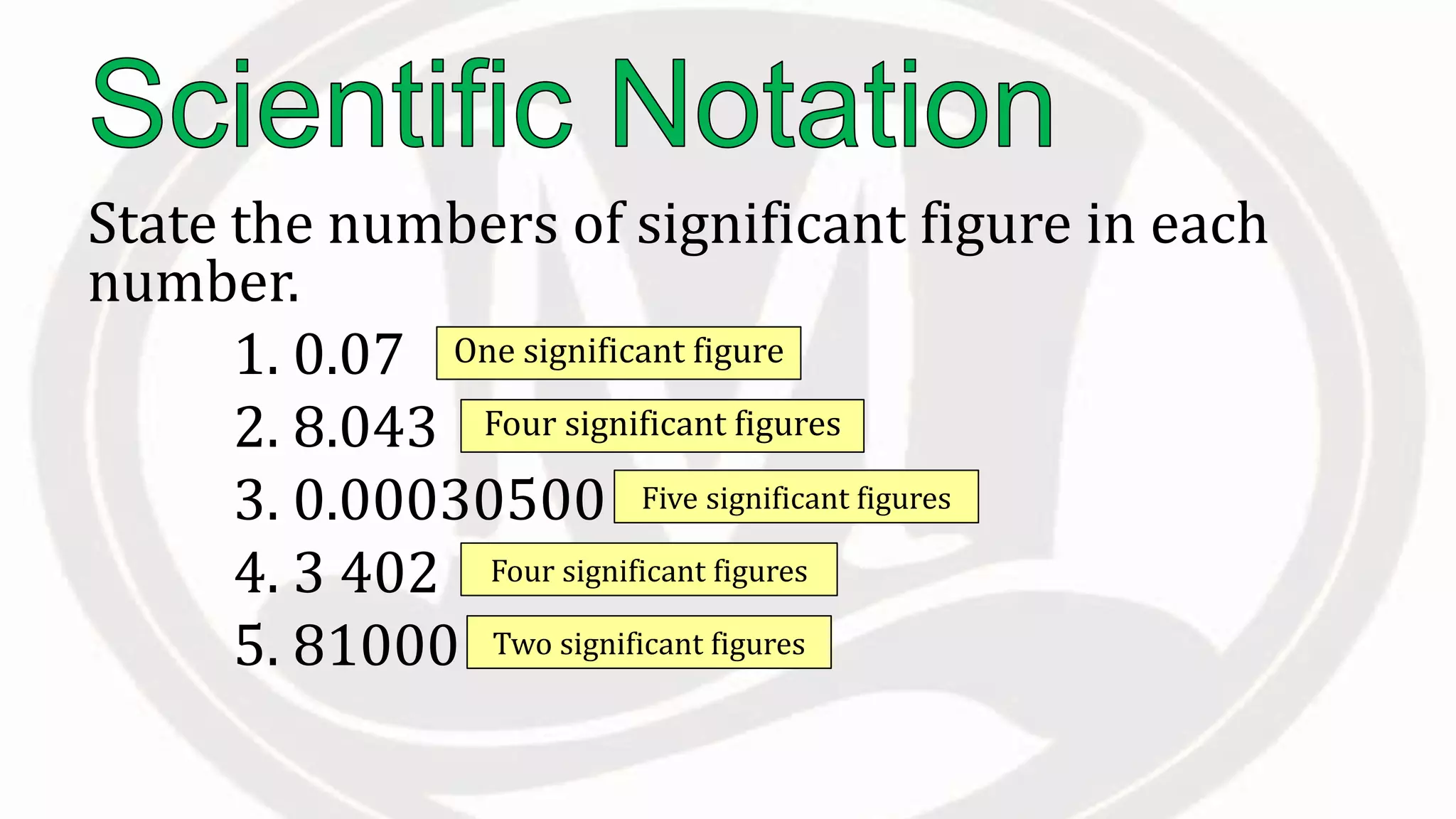
Scientific notation is used to write very large or small numbers in a condensed form. It expresses numbers as the product of a number between 1 and 10 and a power of 10. For example, the distance to the nearest star can be written as 24,700,000,000,000 miles, which in scientific notation is 2.47 × 1013 miles. A single bacteria is about 0.00000075 meters in diameter, which is 7.5 × 10-7 meters in scientific notation. The document then provides examples and steps for converting numbers between standard decimal form and scientific notation.