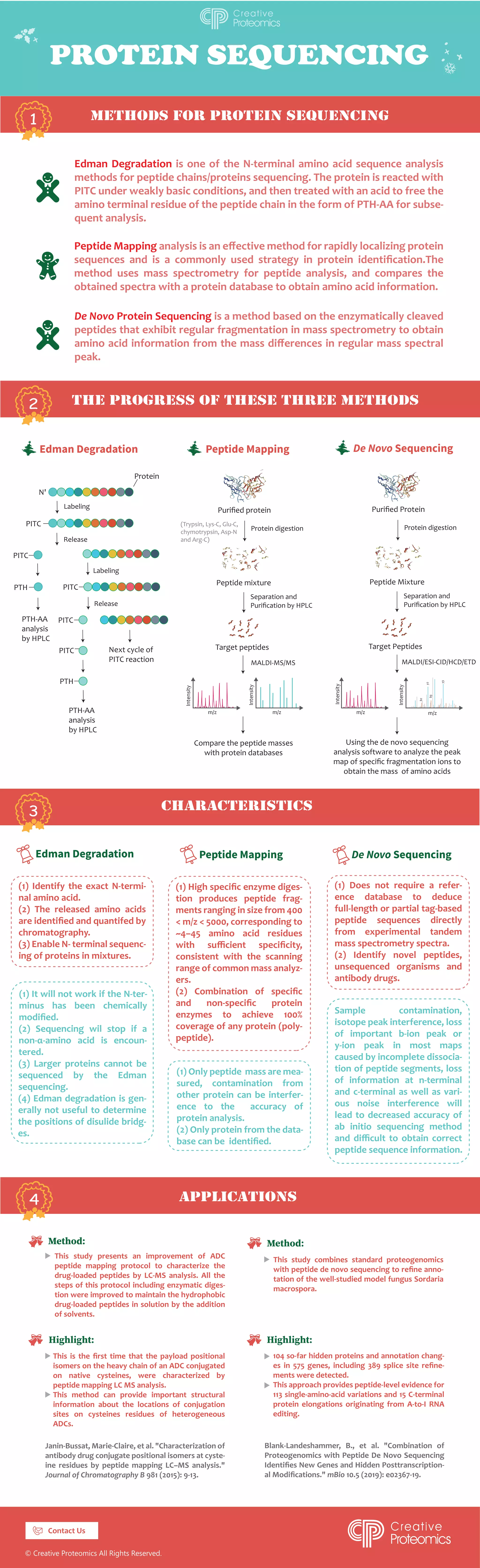
The document discusses various protein sequencing methods, including Edman degradation, peptide mapping analysis using mass spectrometry, and de novo protein sequencing based on enzymatic cleavage. Each method has specific applications, strengths, and limitations, such as the accuracy of amino acid identification and the ability to sequence larger proteins. Additionally, improvements in peptide mapping protocols for antibody drug conjugates are mentioned, highlighting their importance in understanding conjugation sites and structural information.