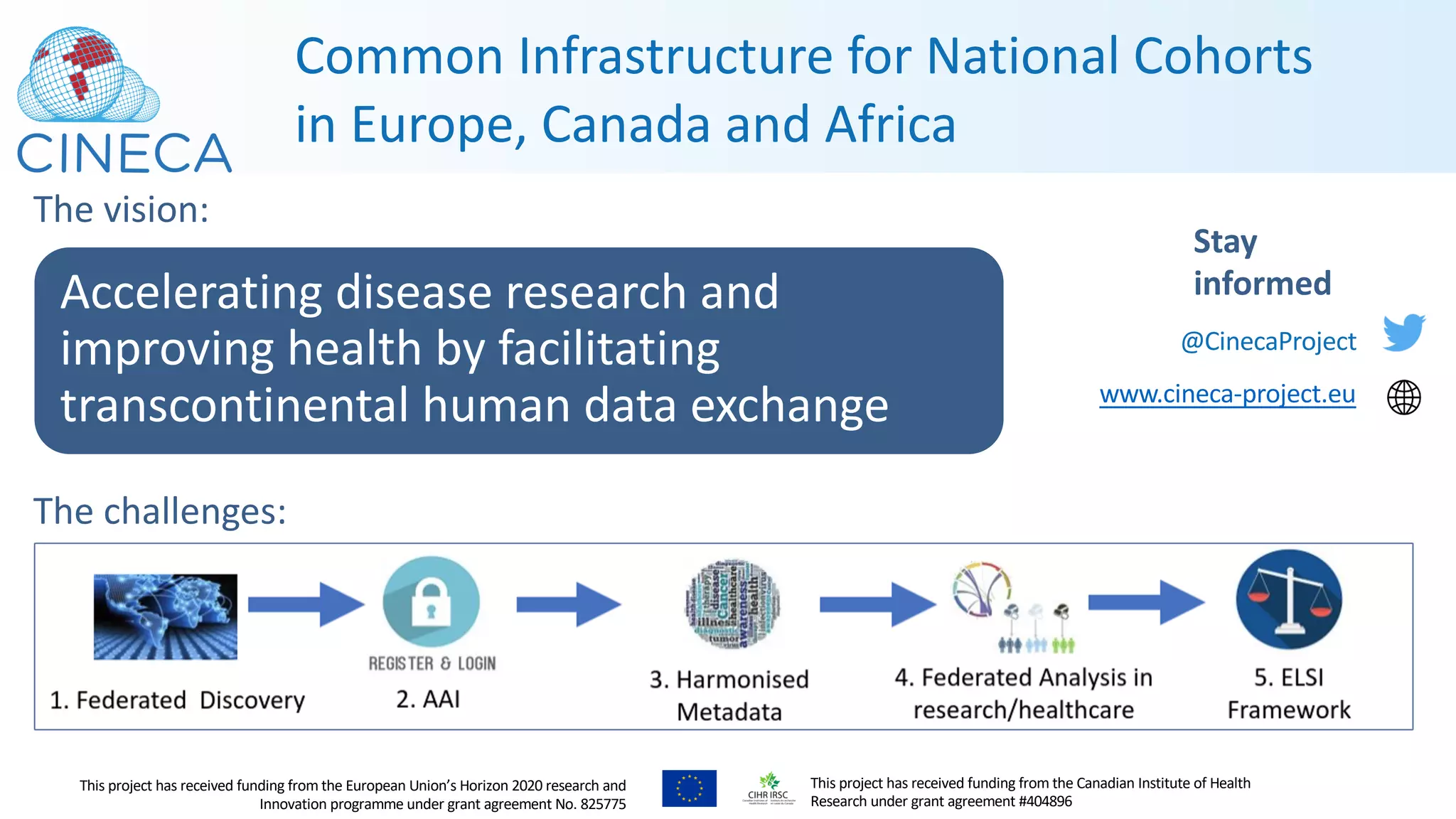
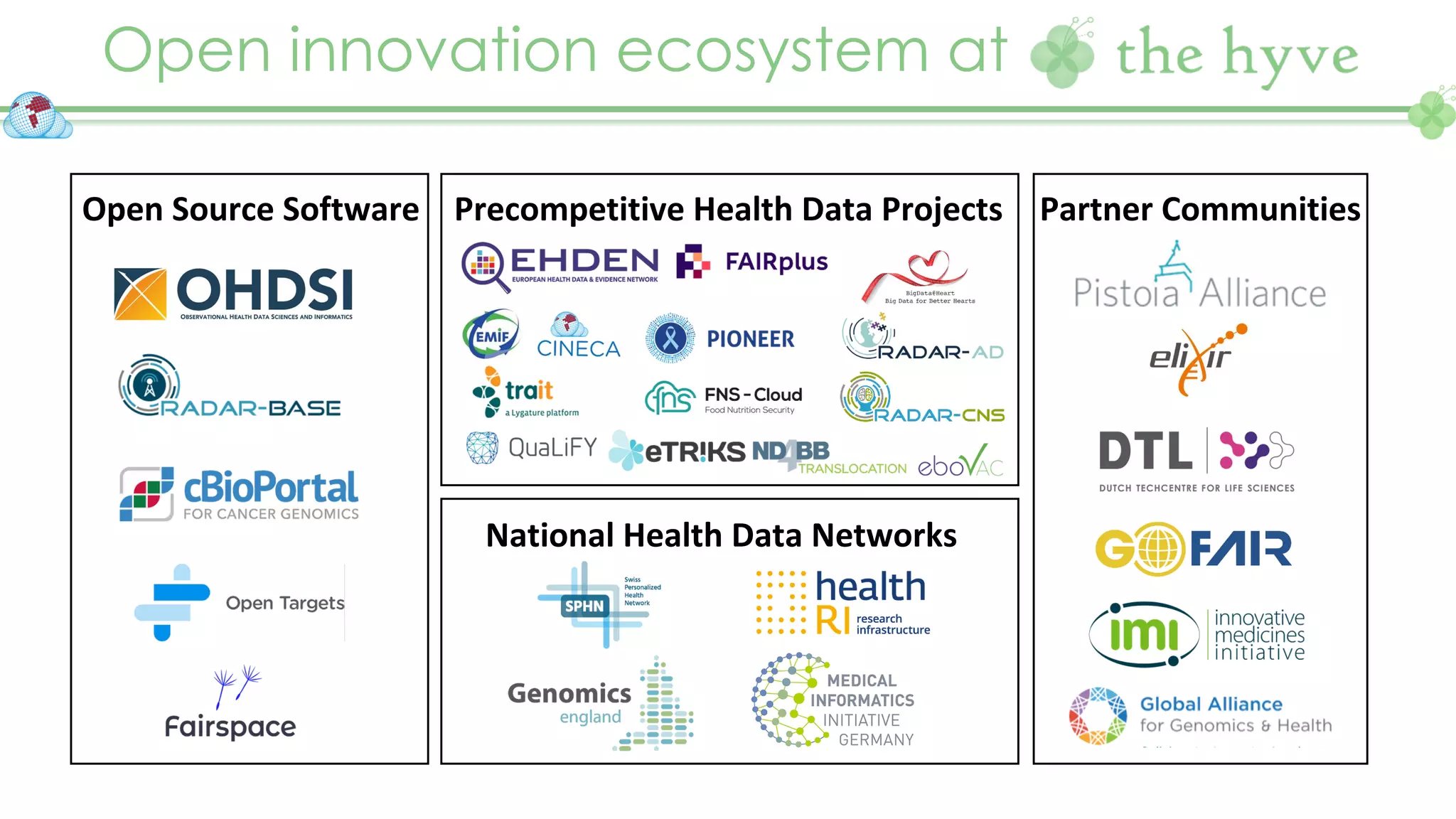
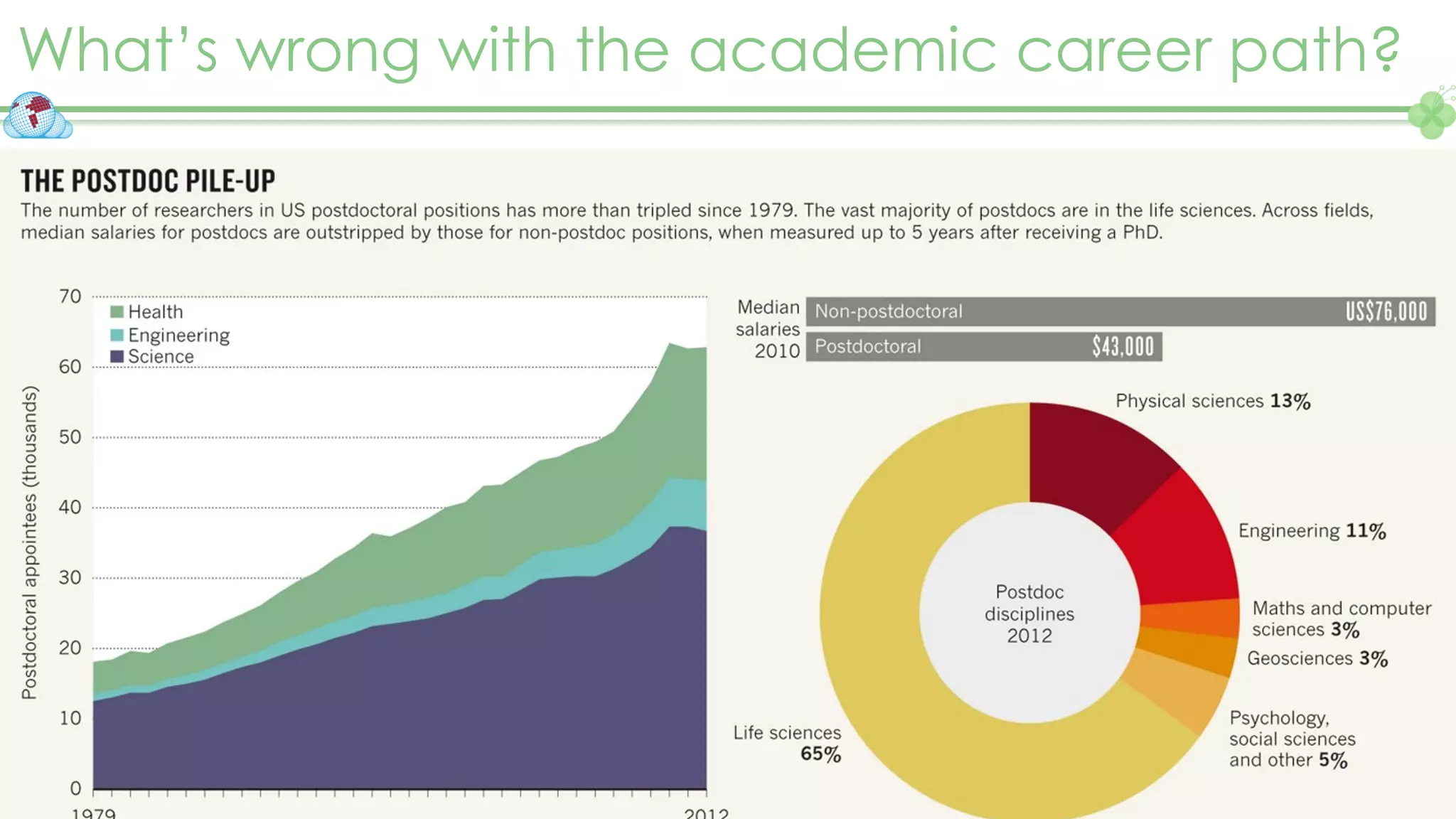
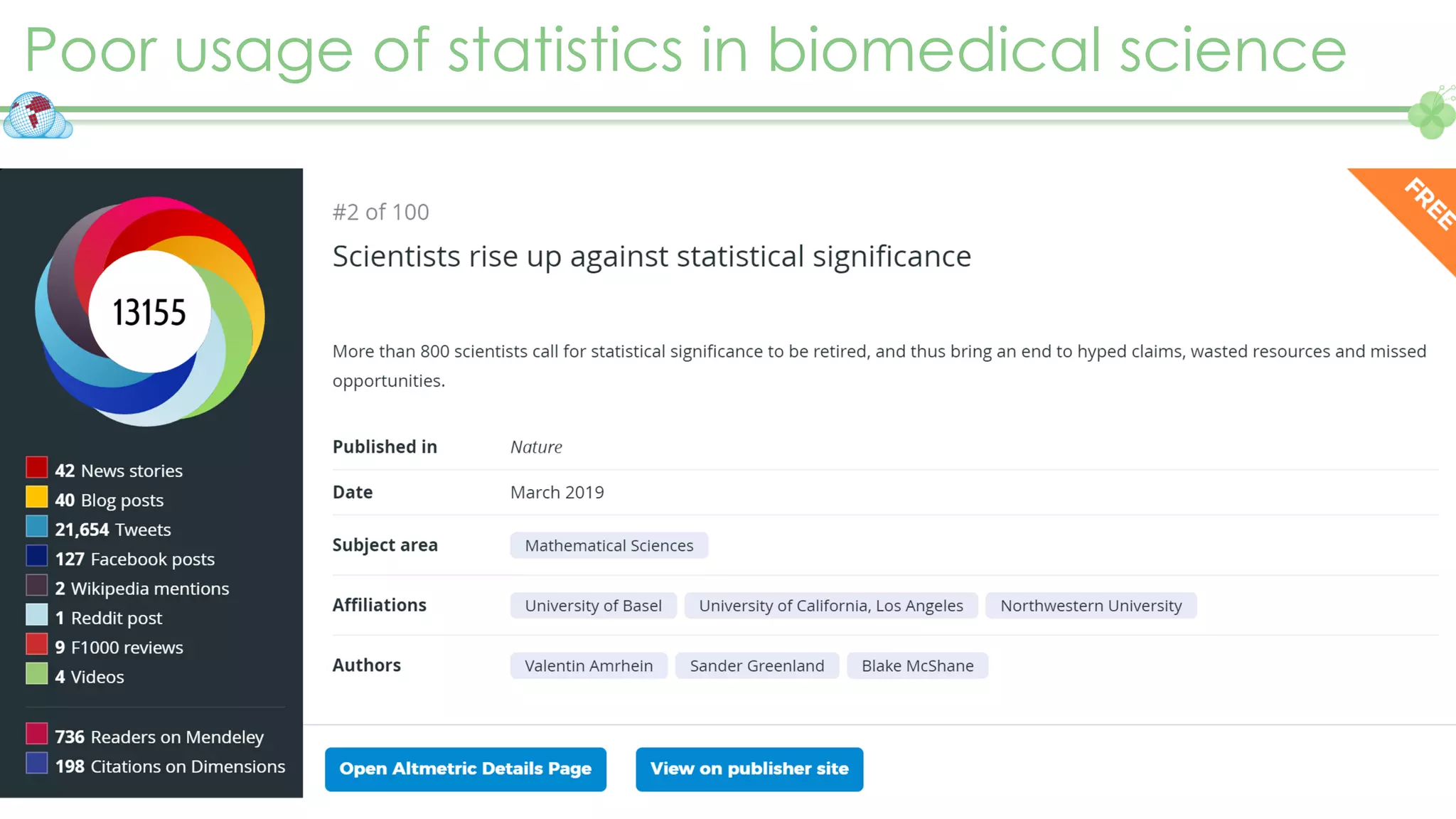
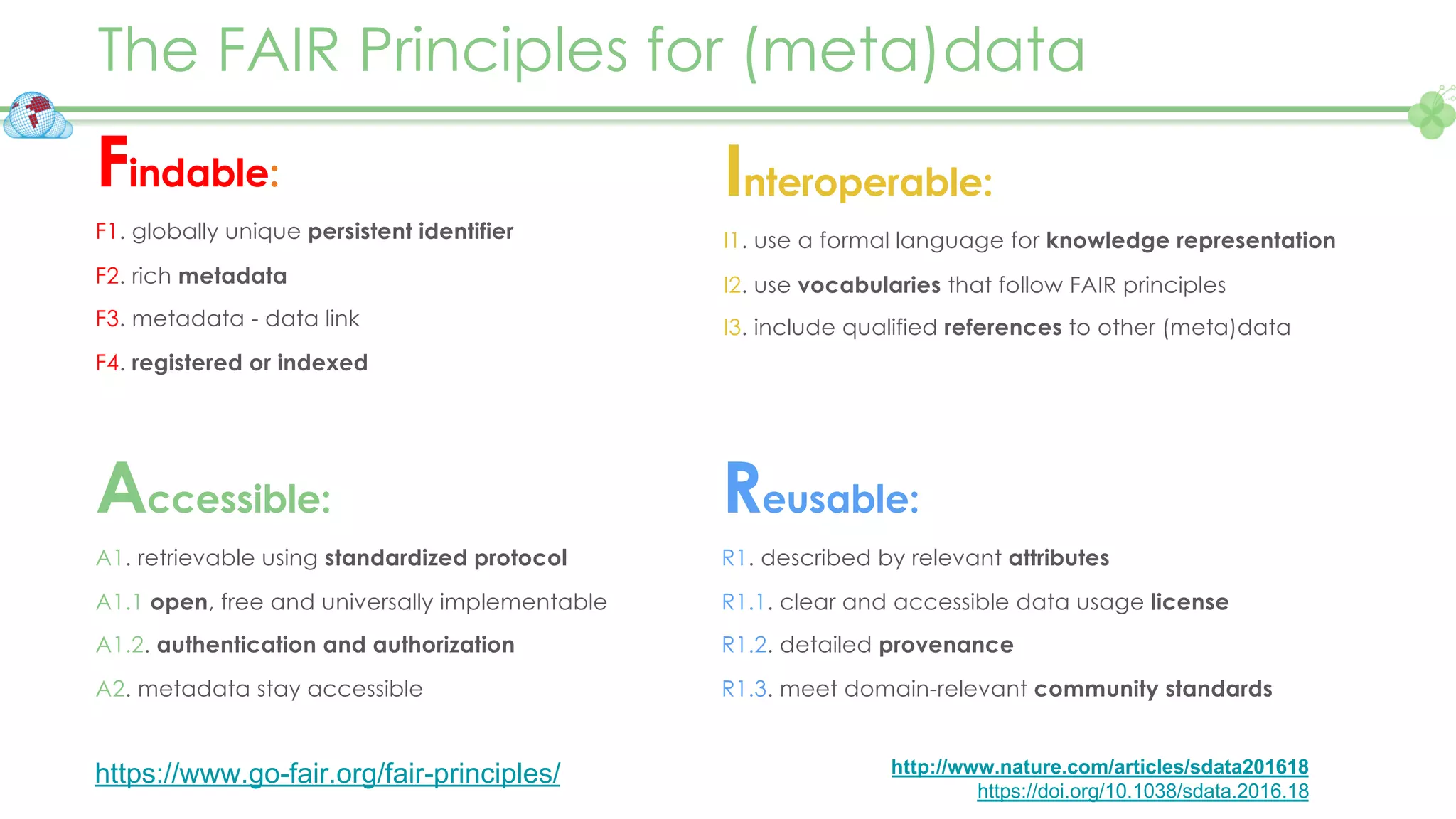
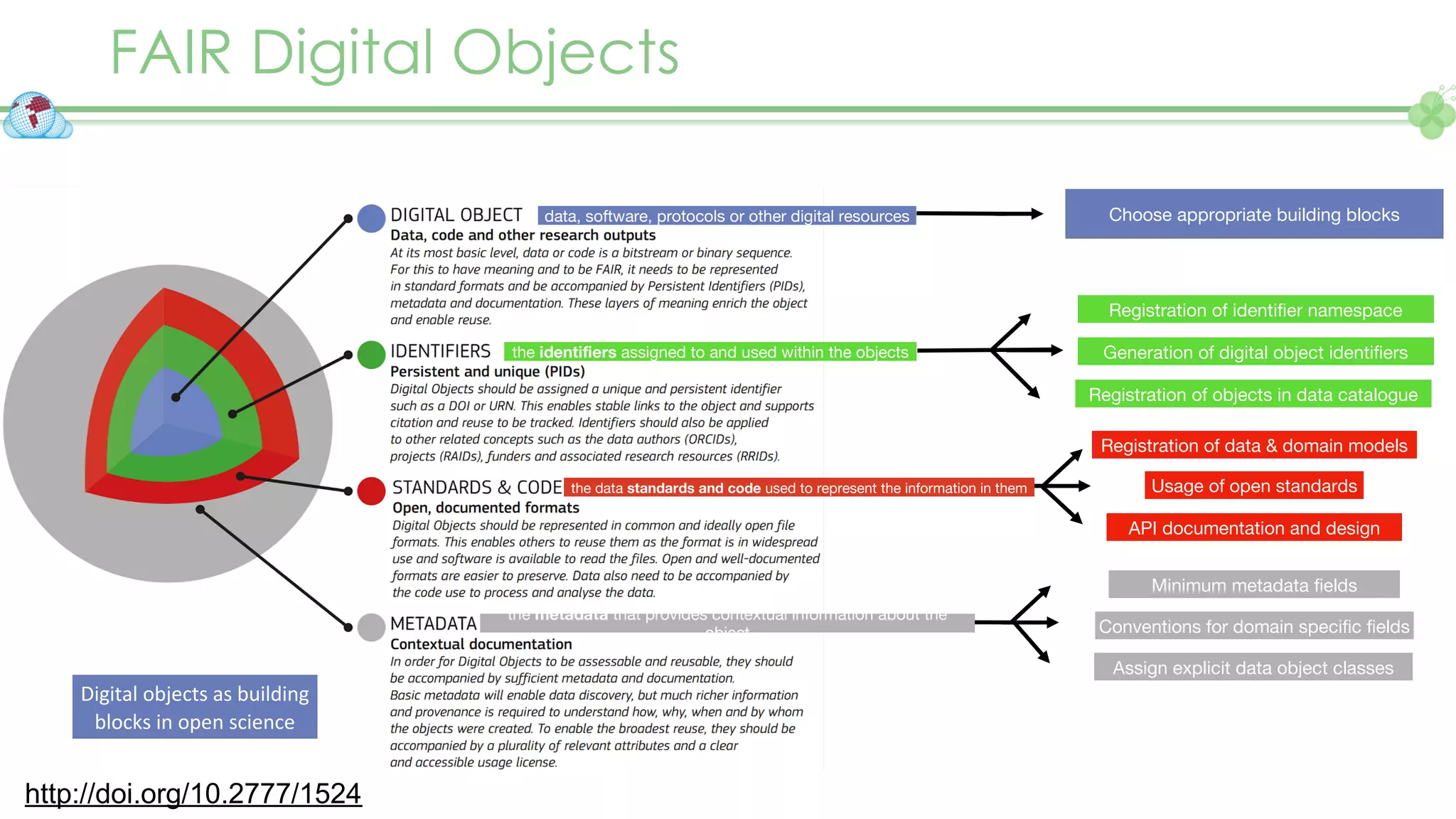
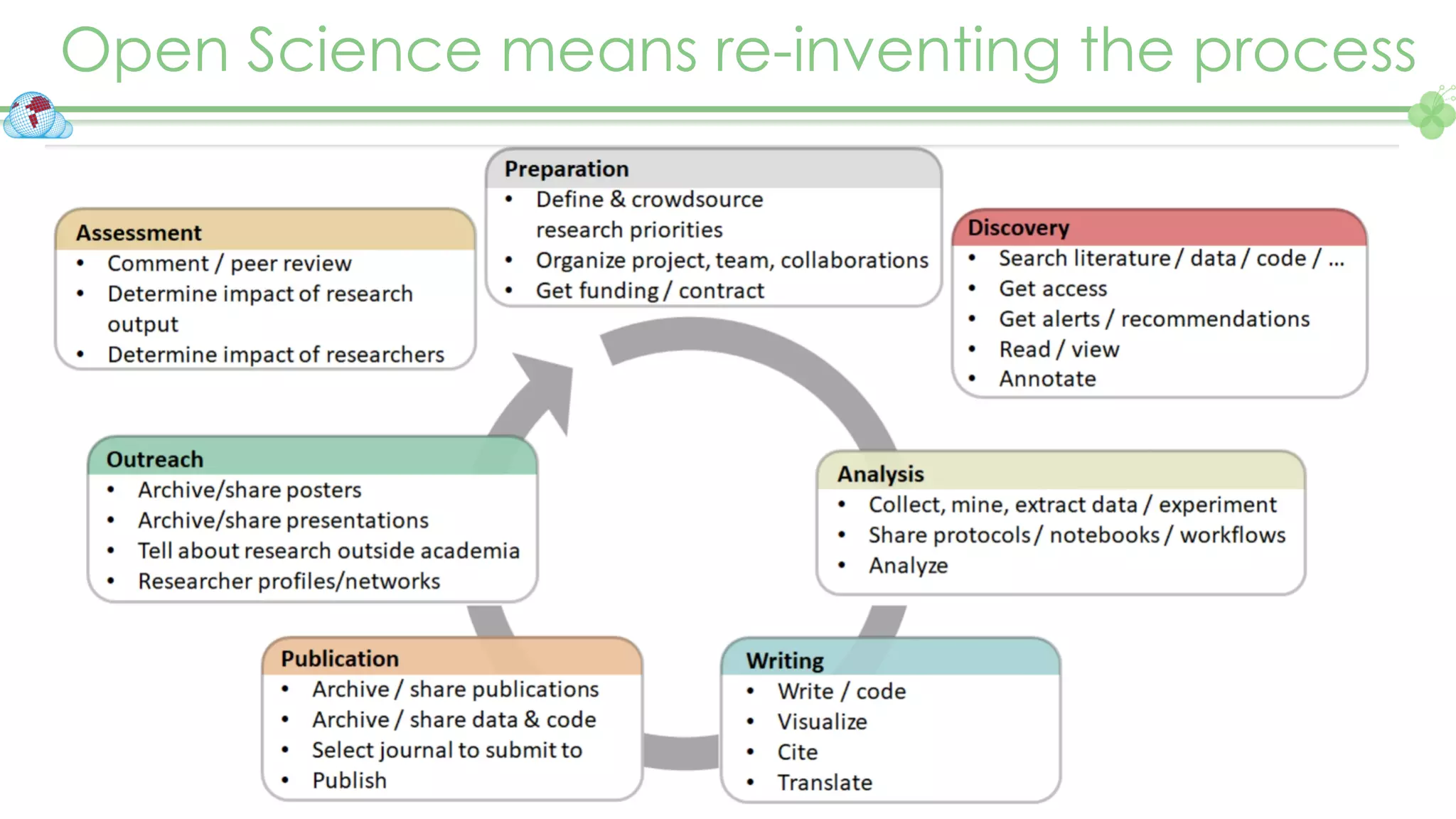
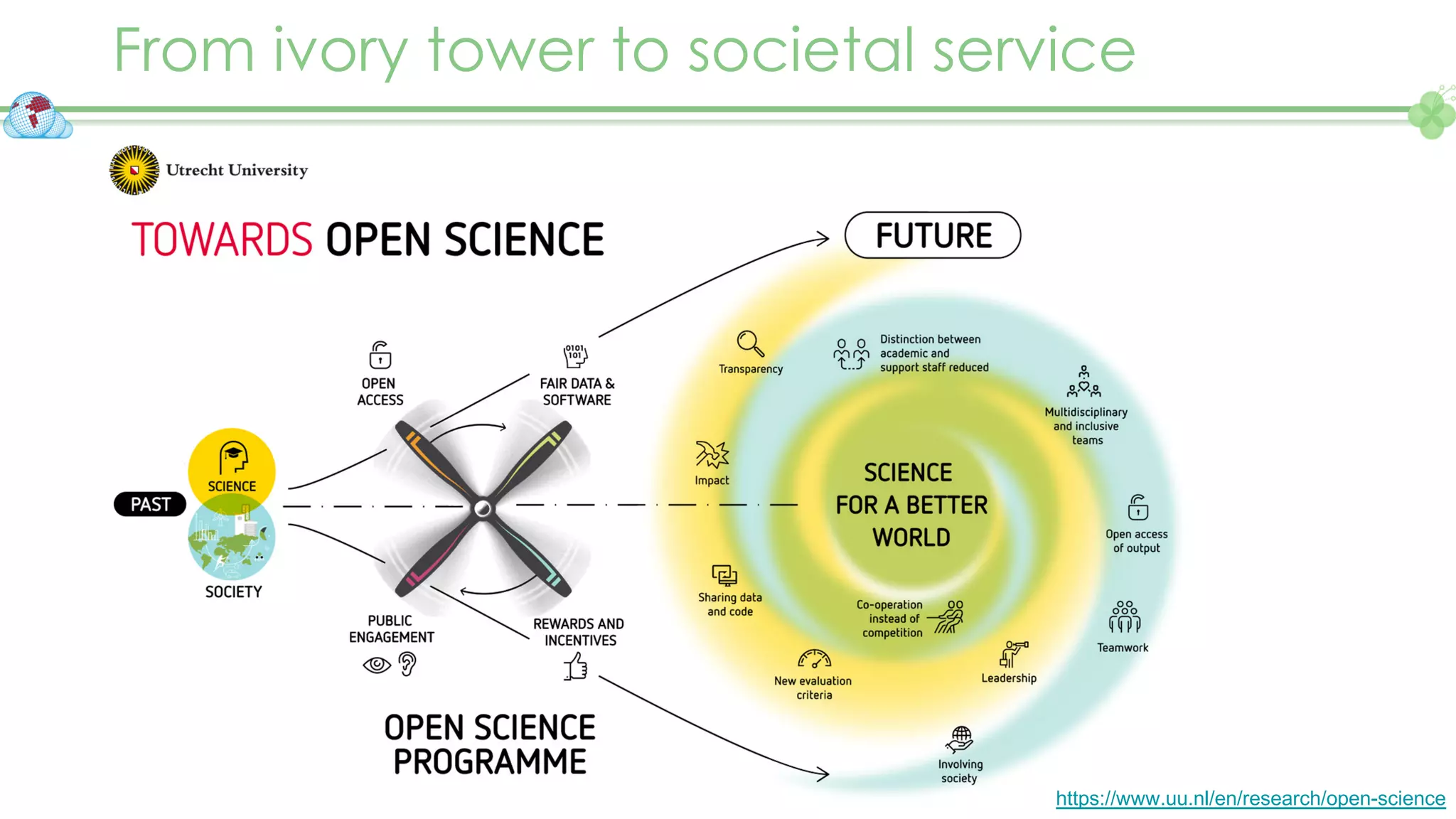
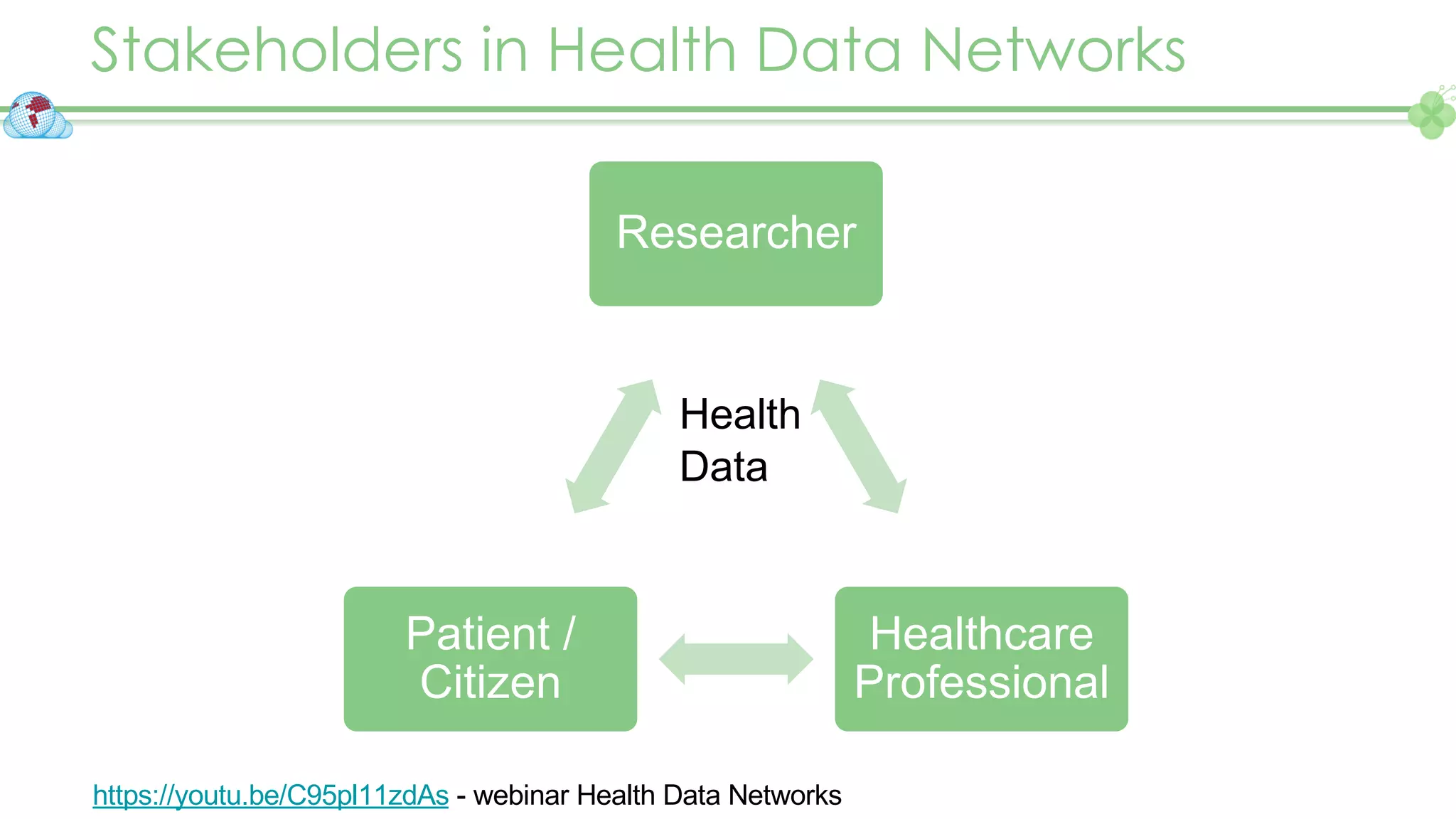
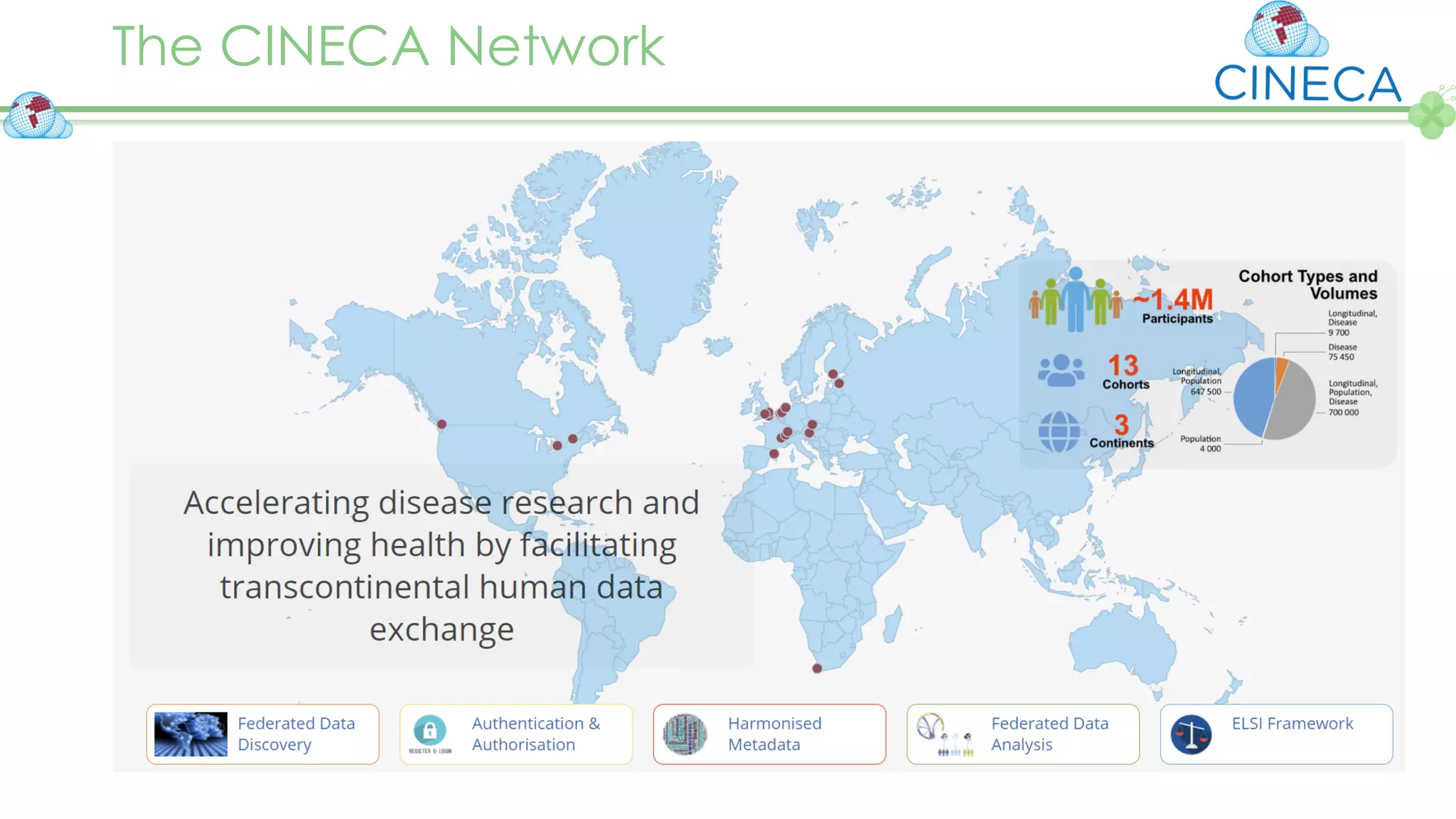
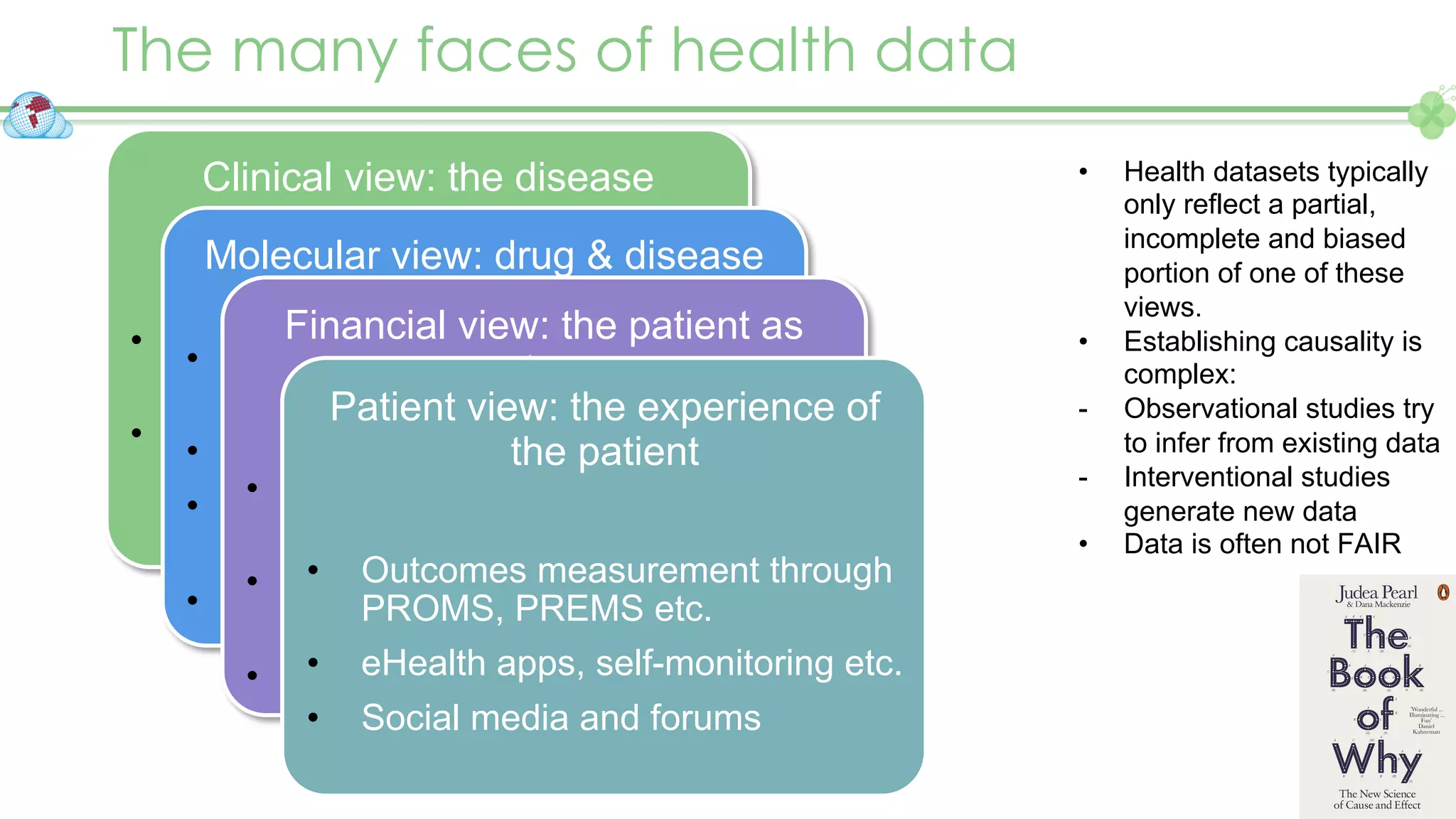
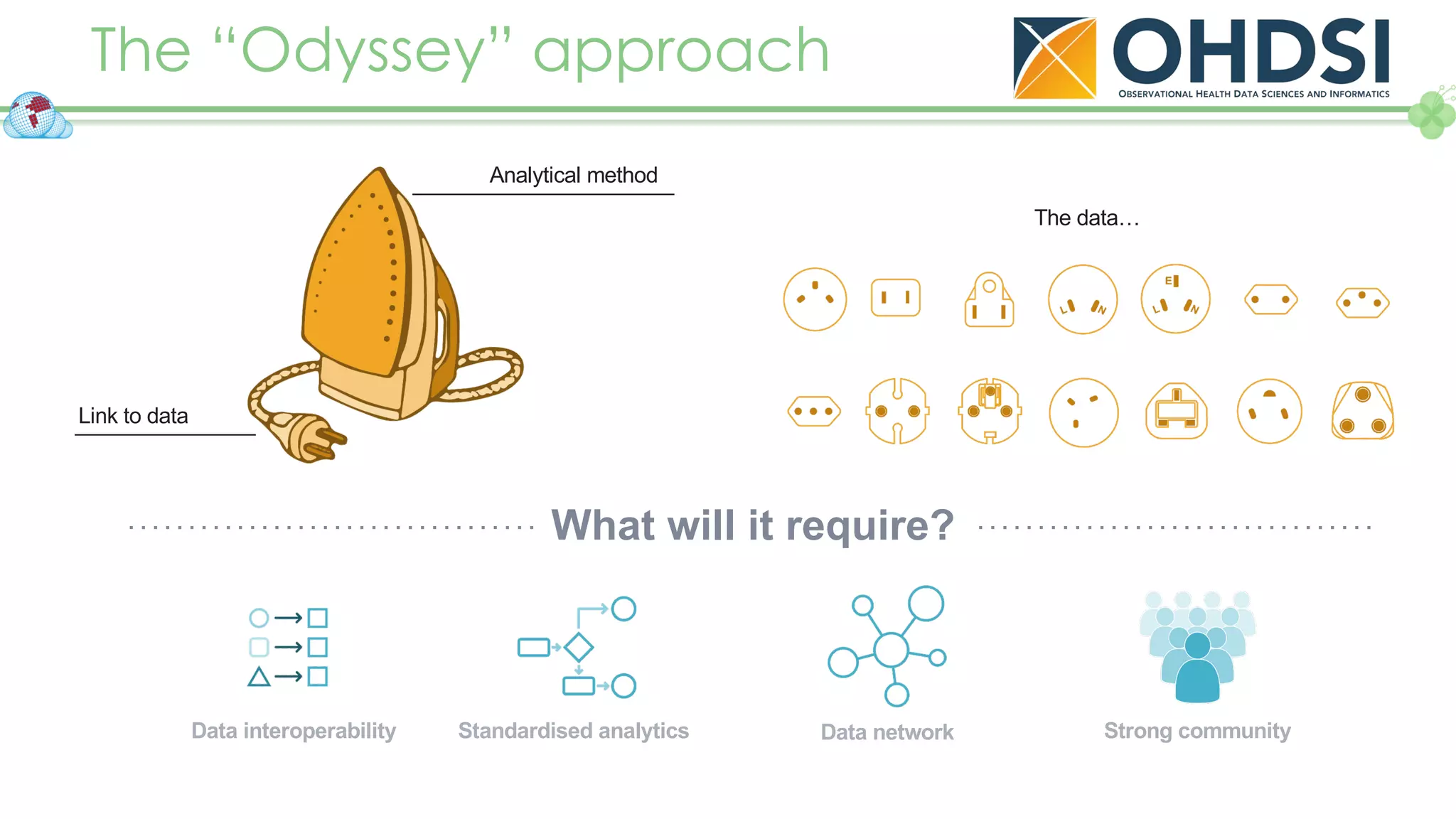
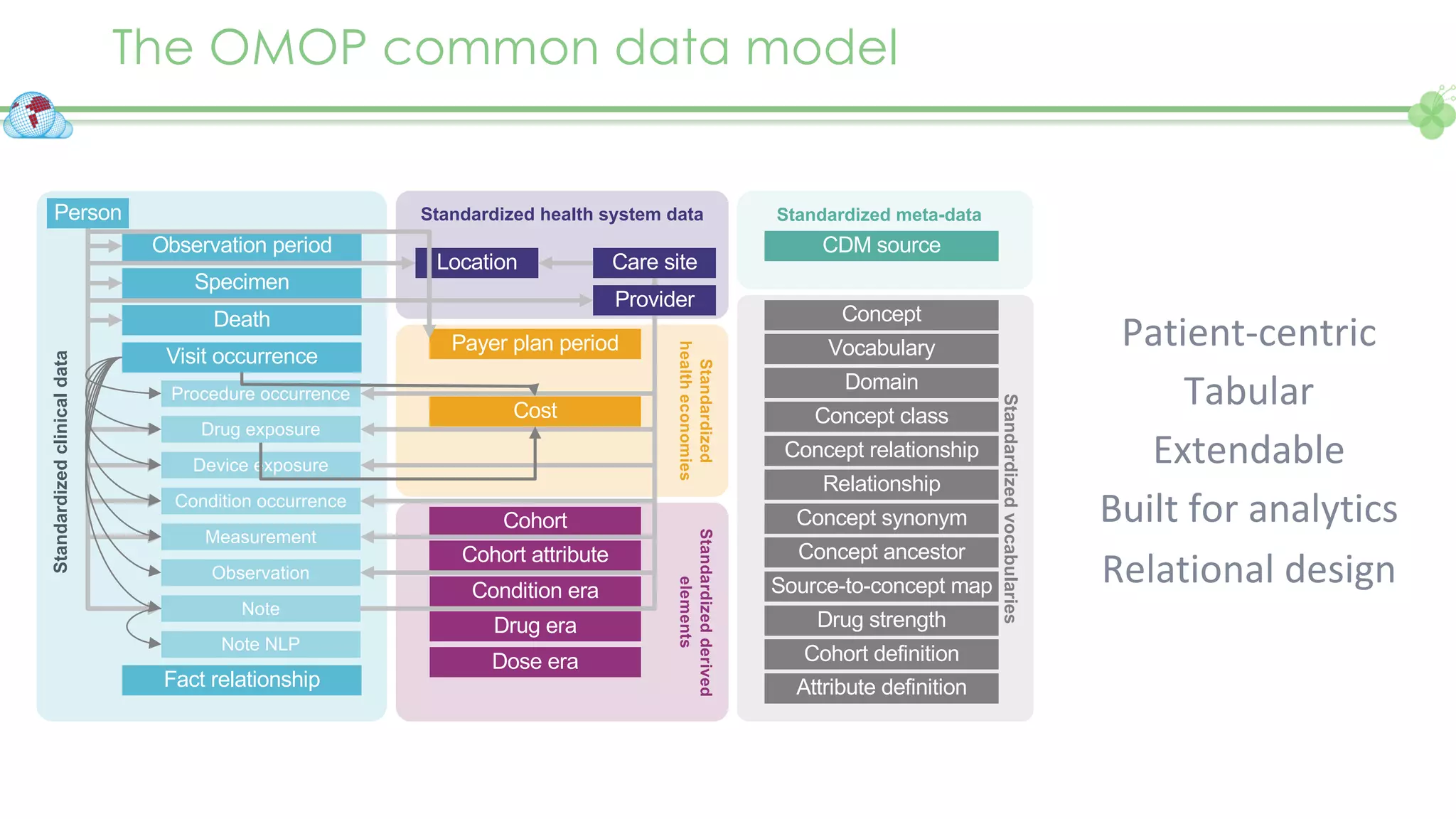
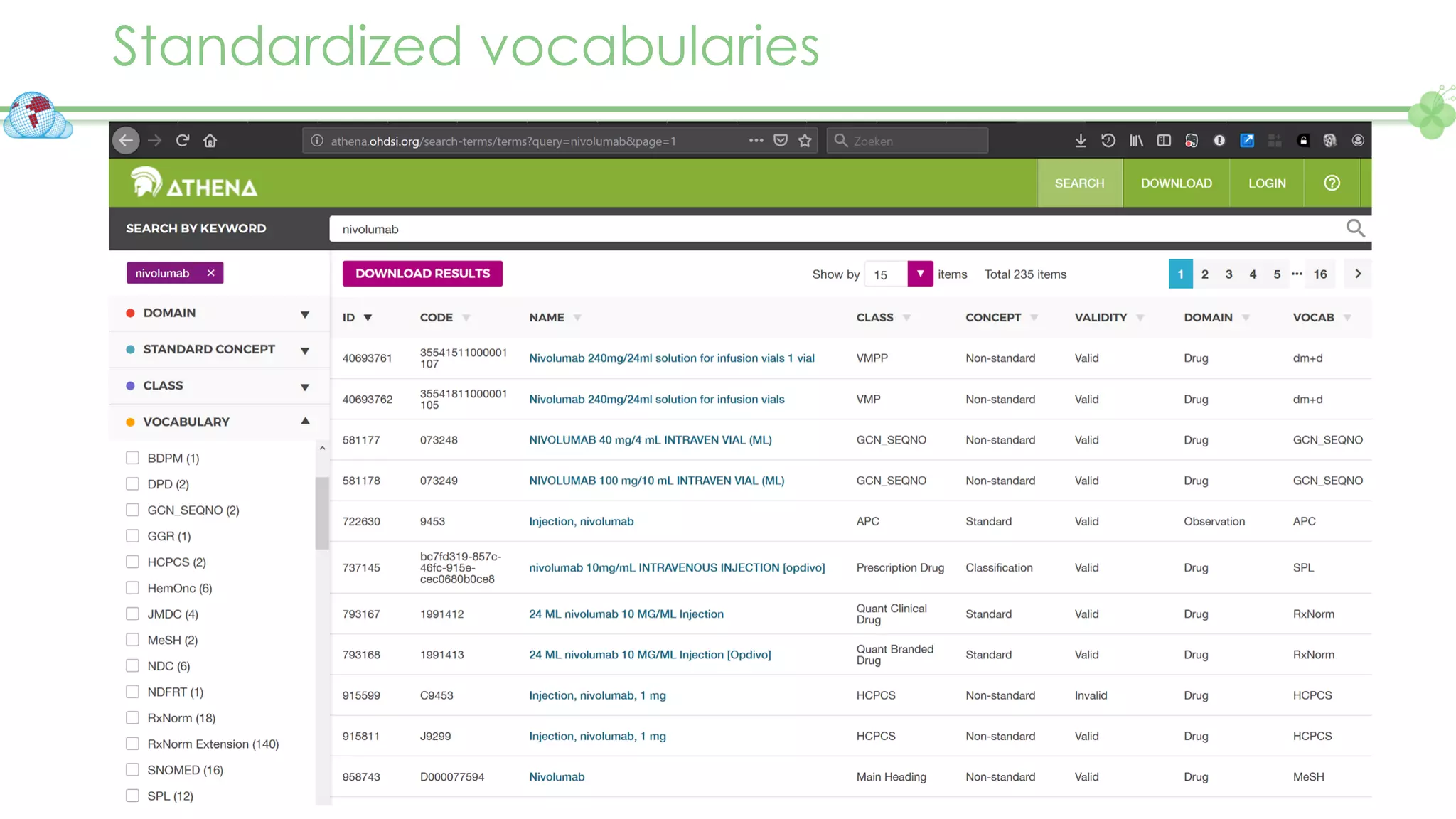
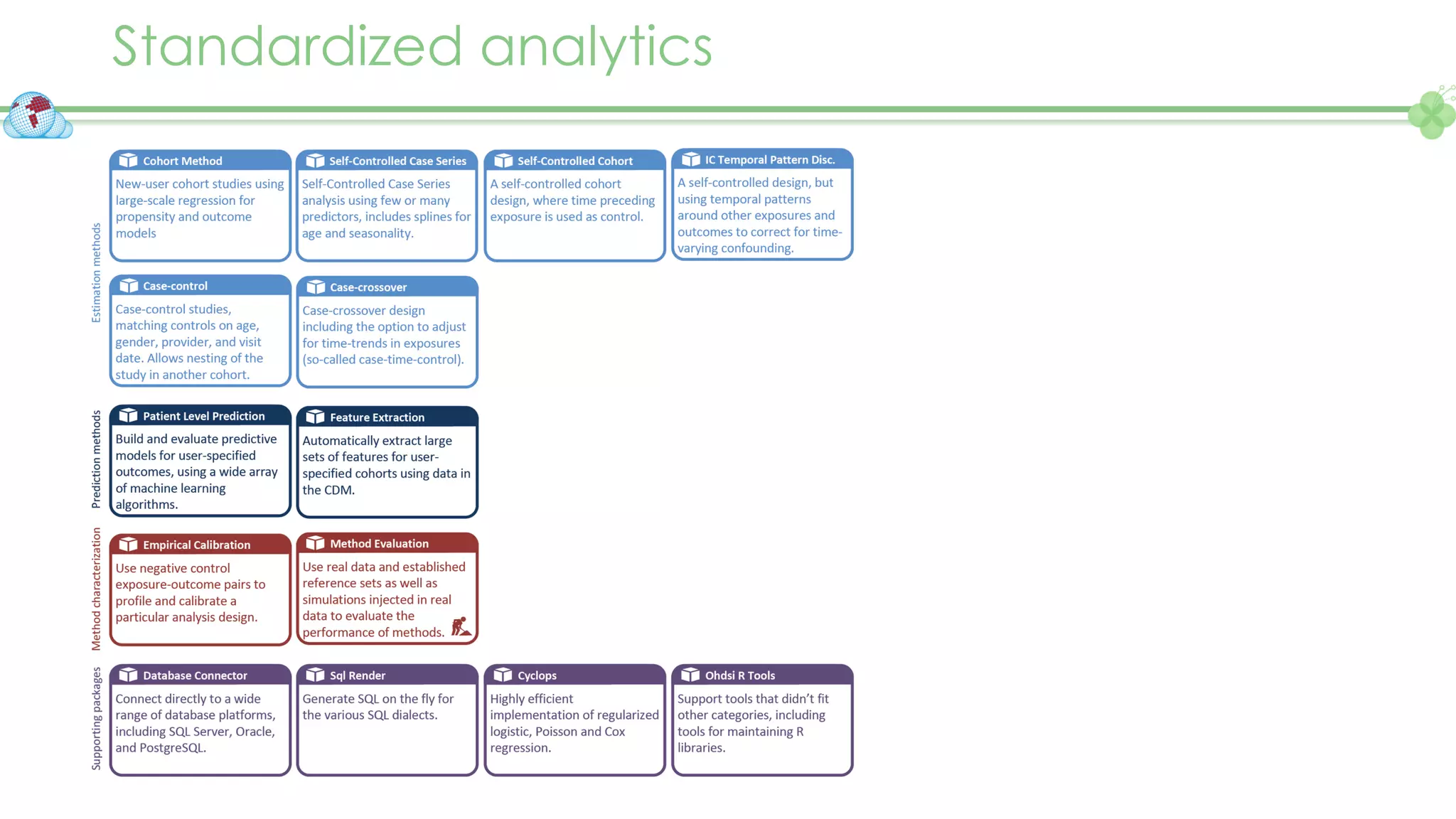
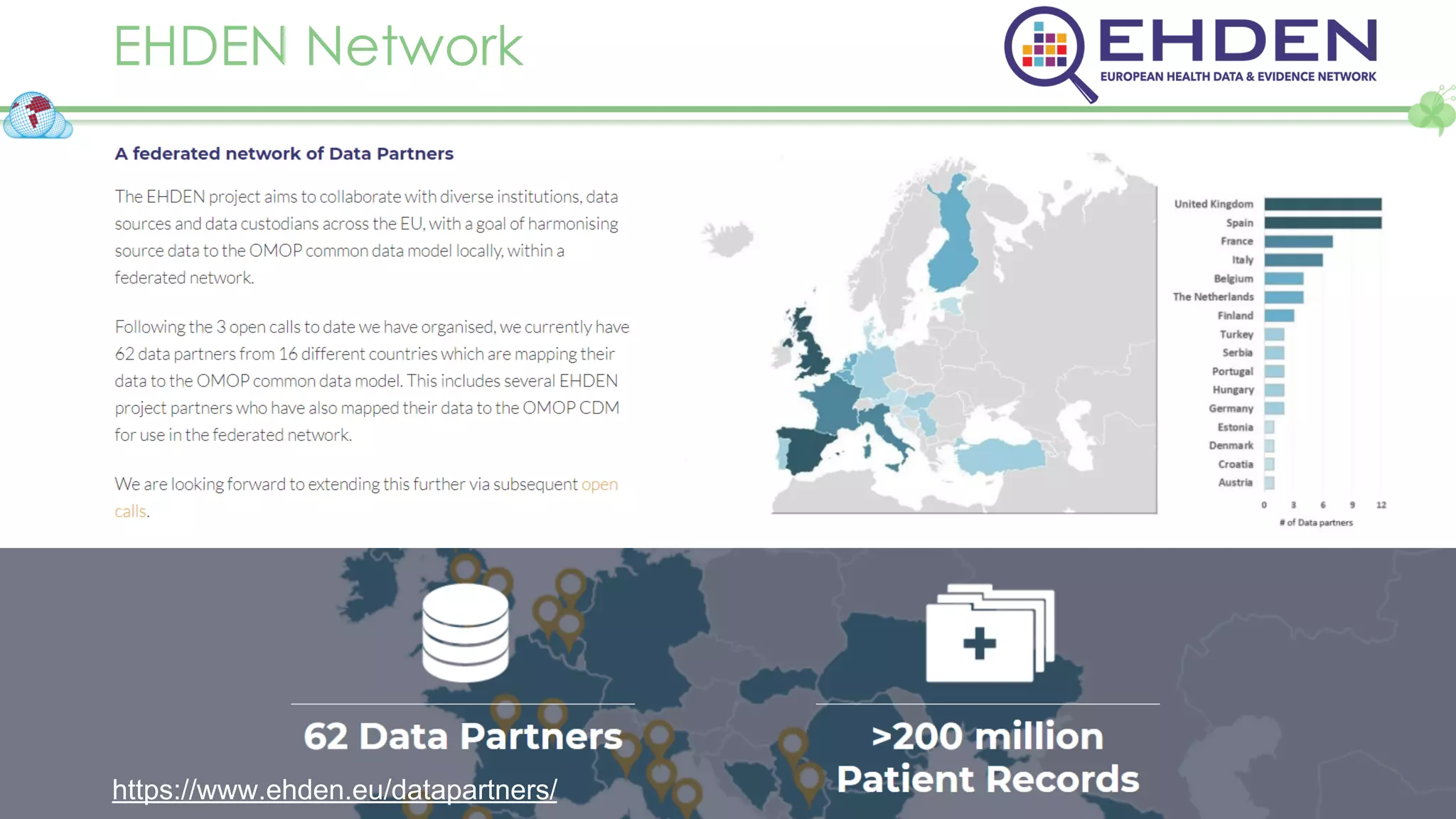
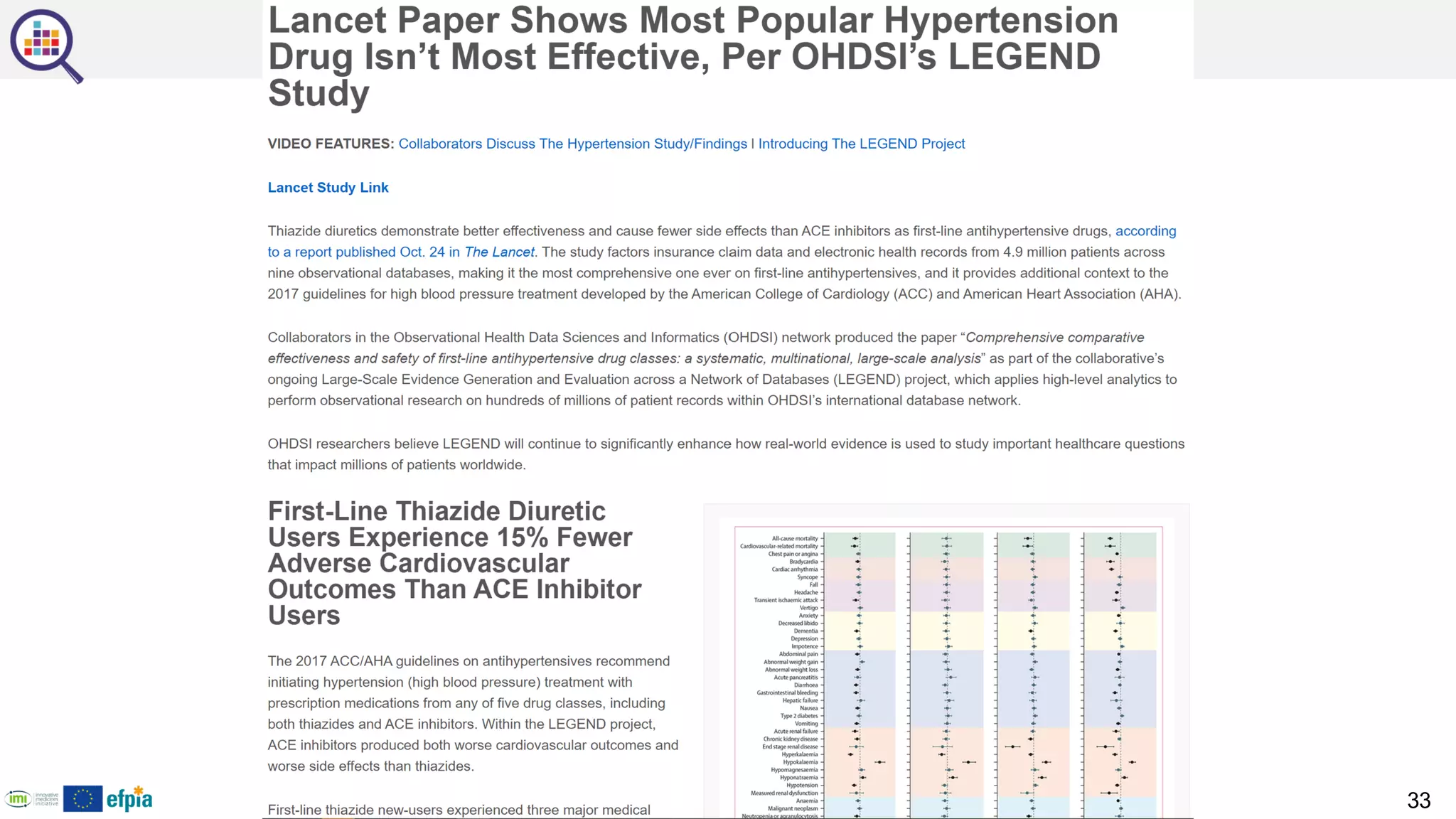
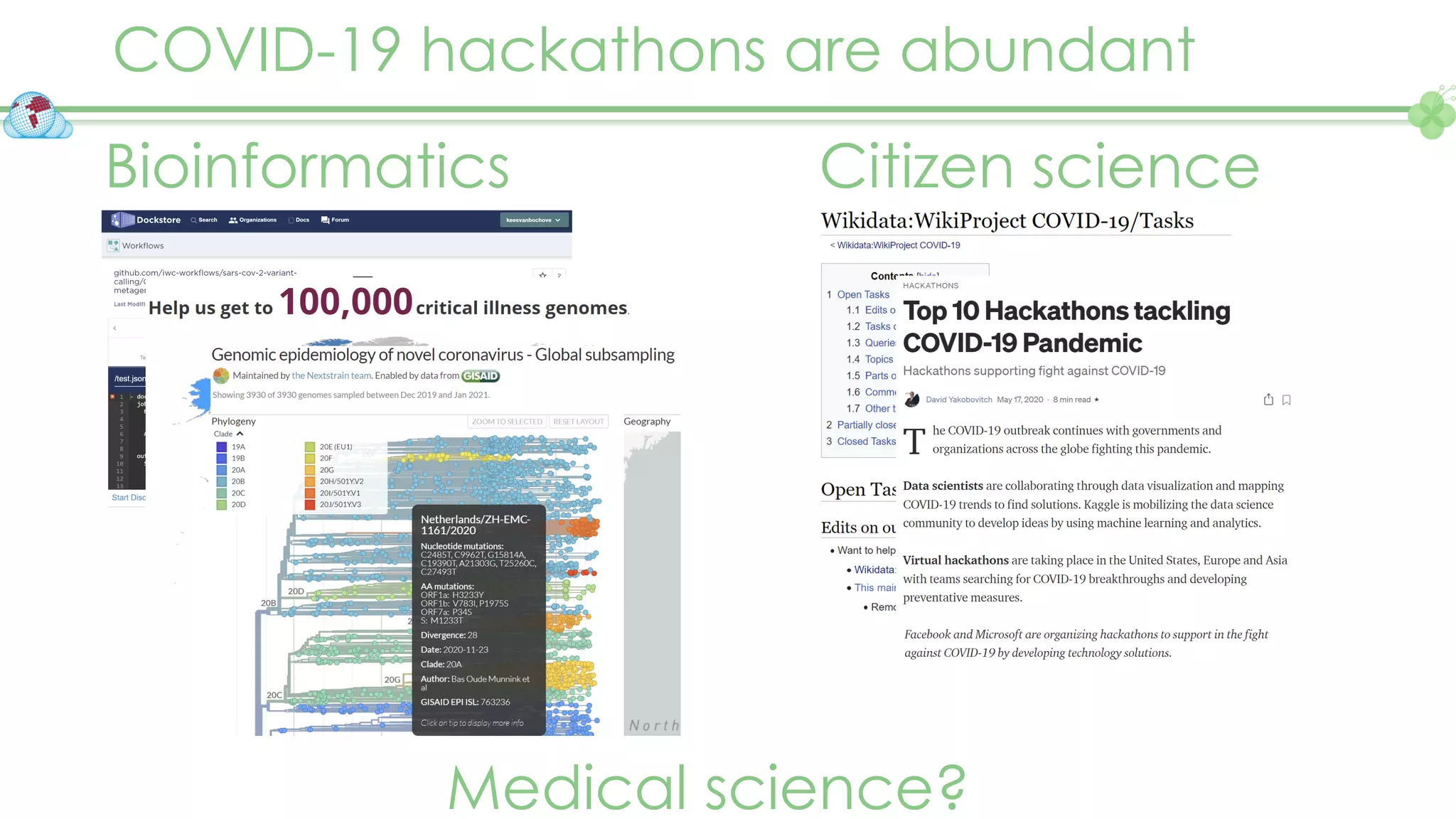
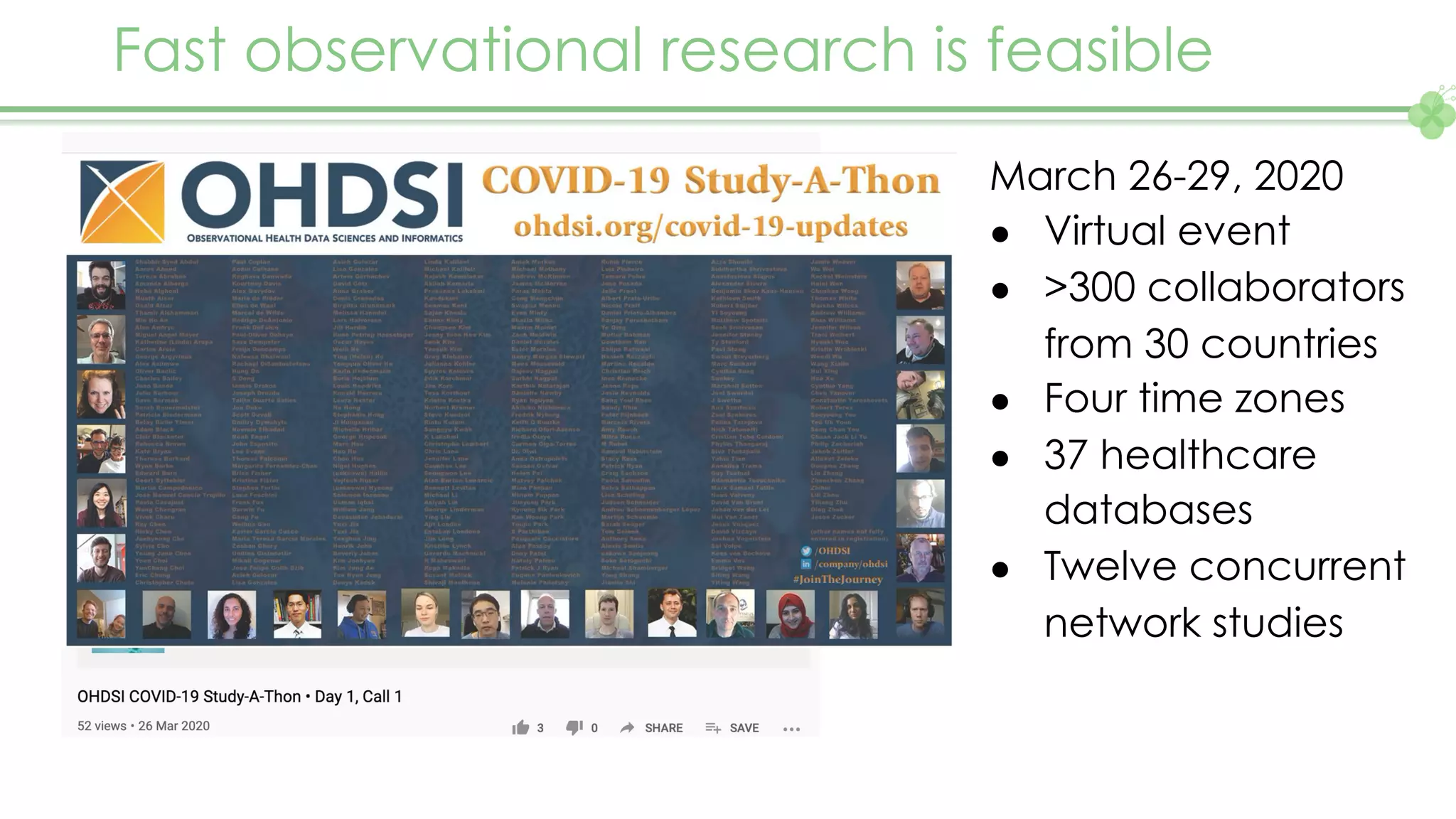
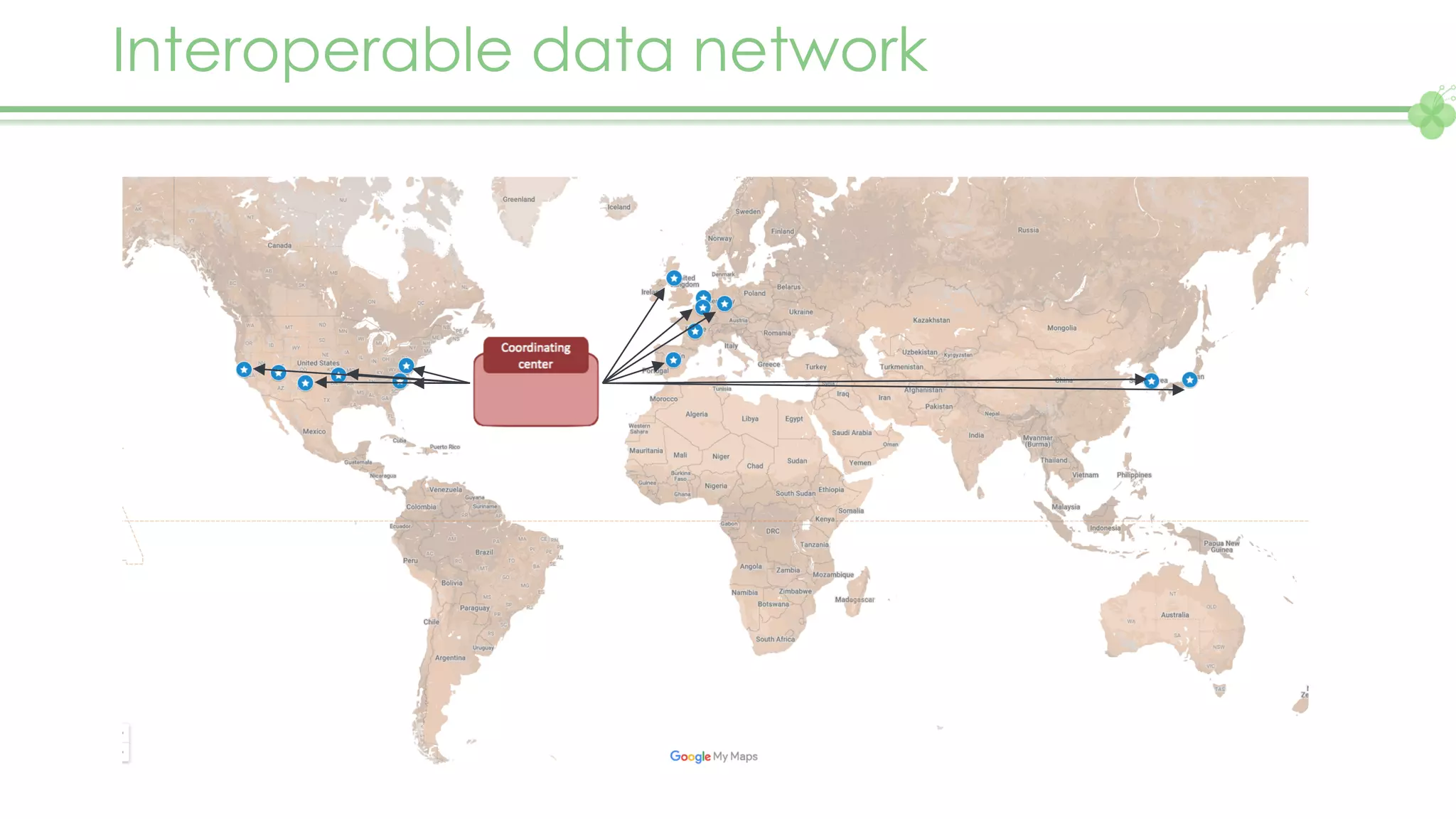
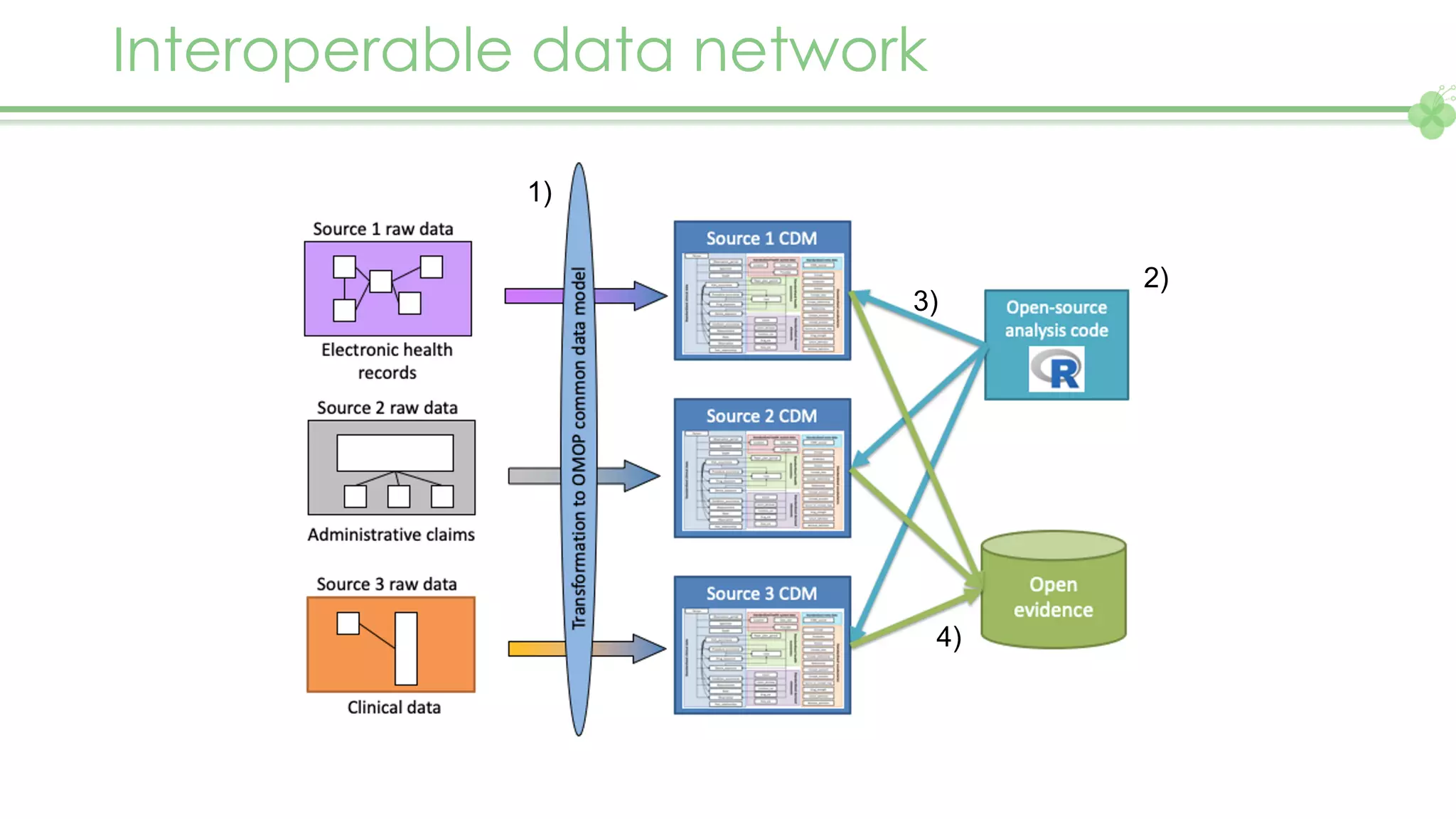
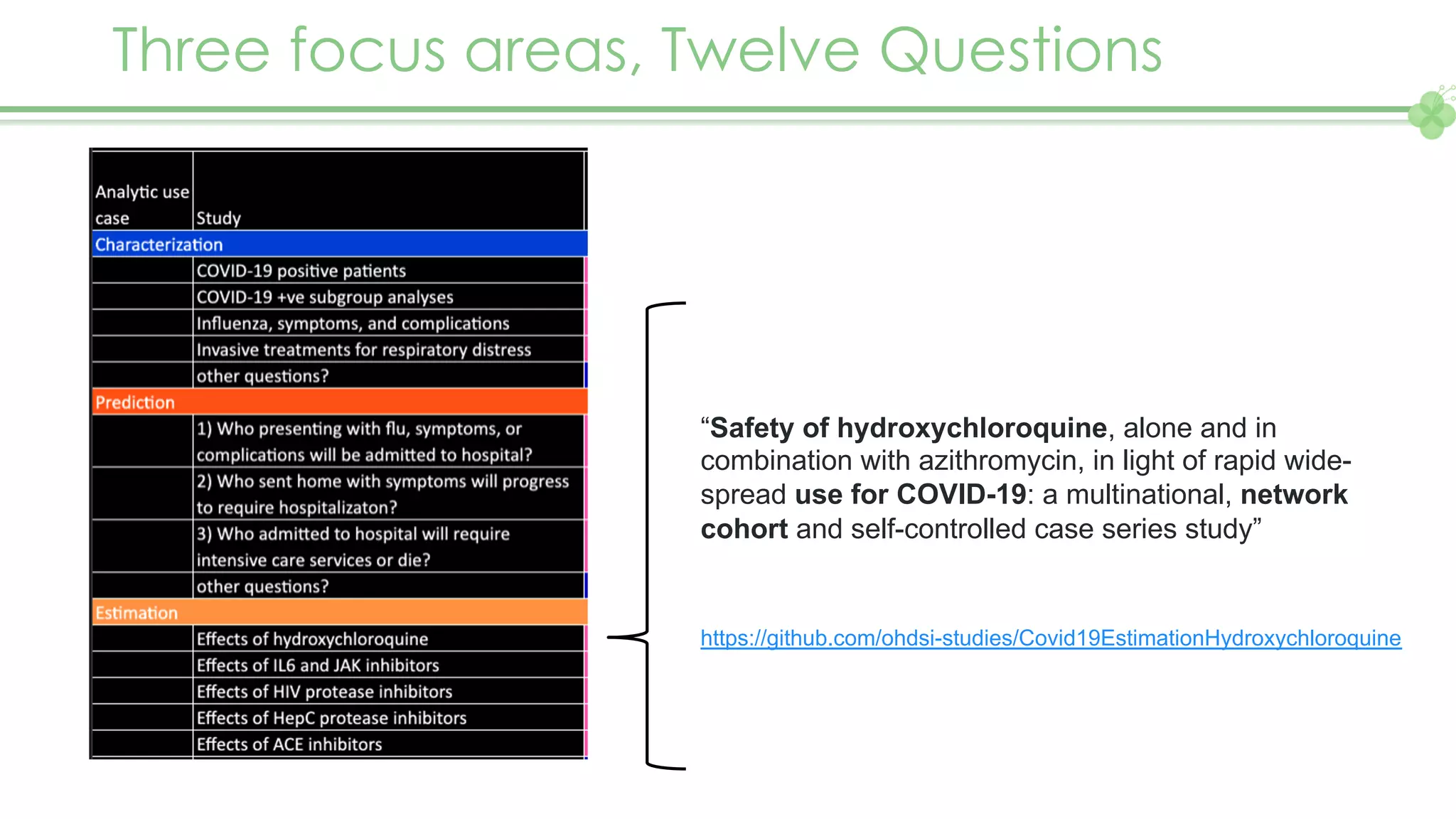
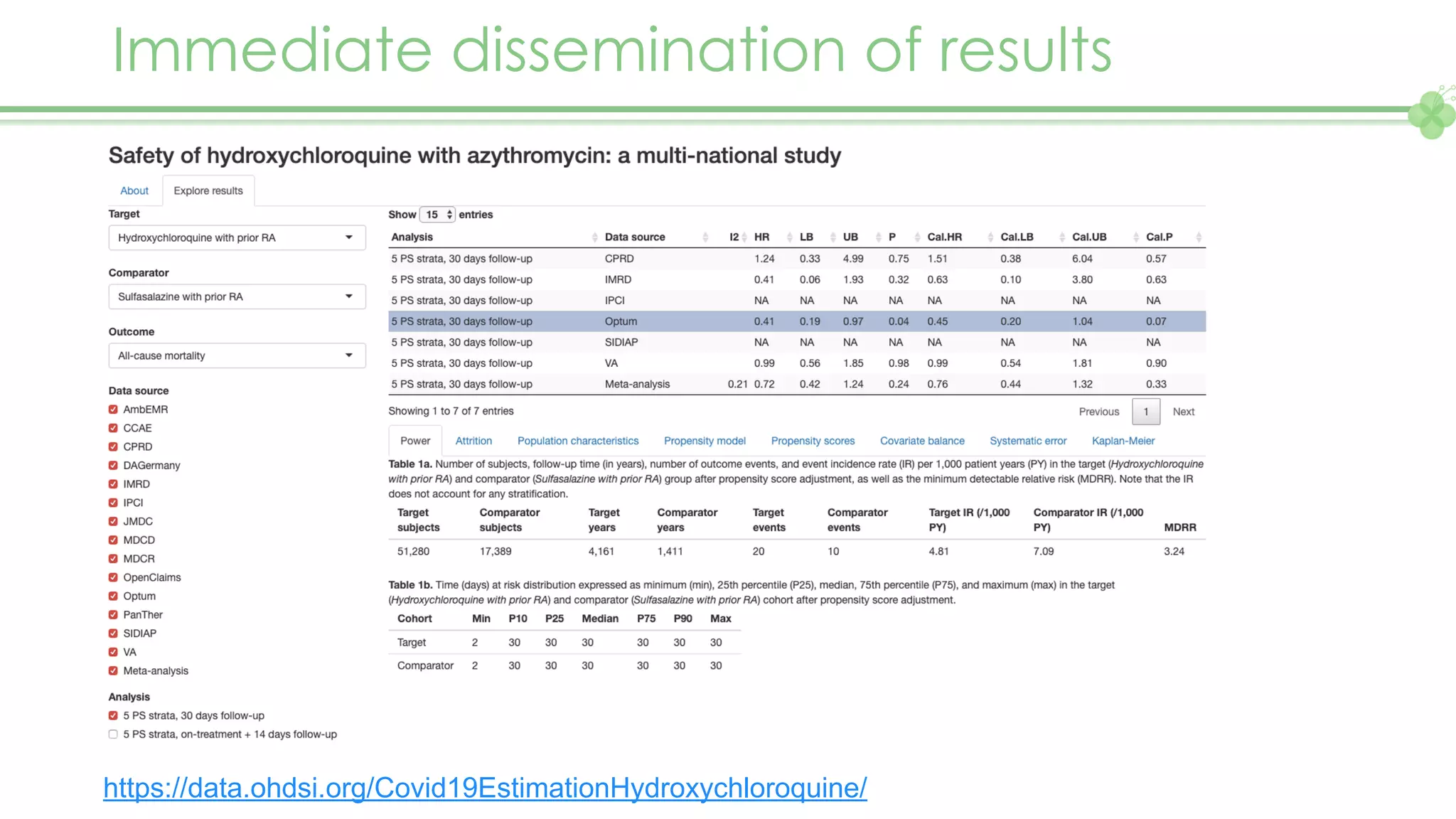
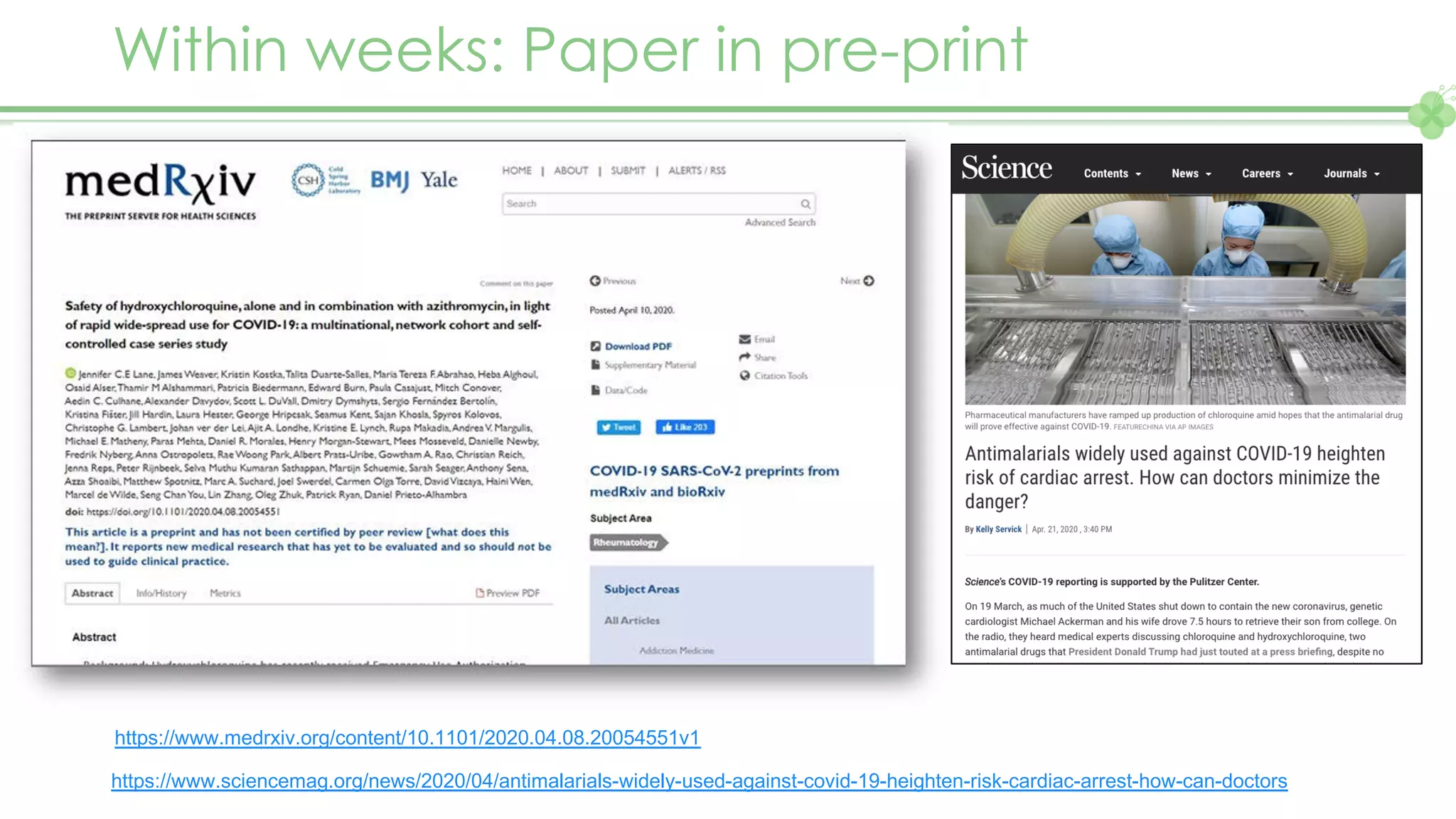
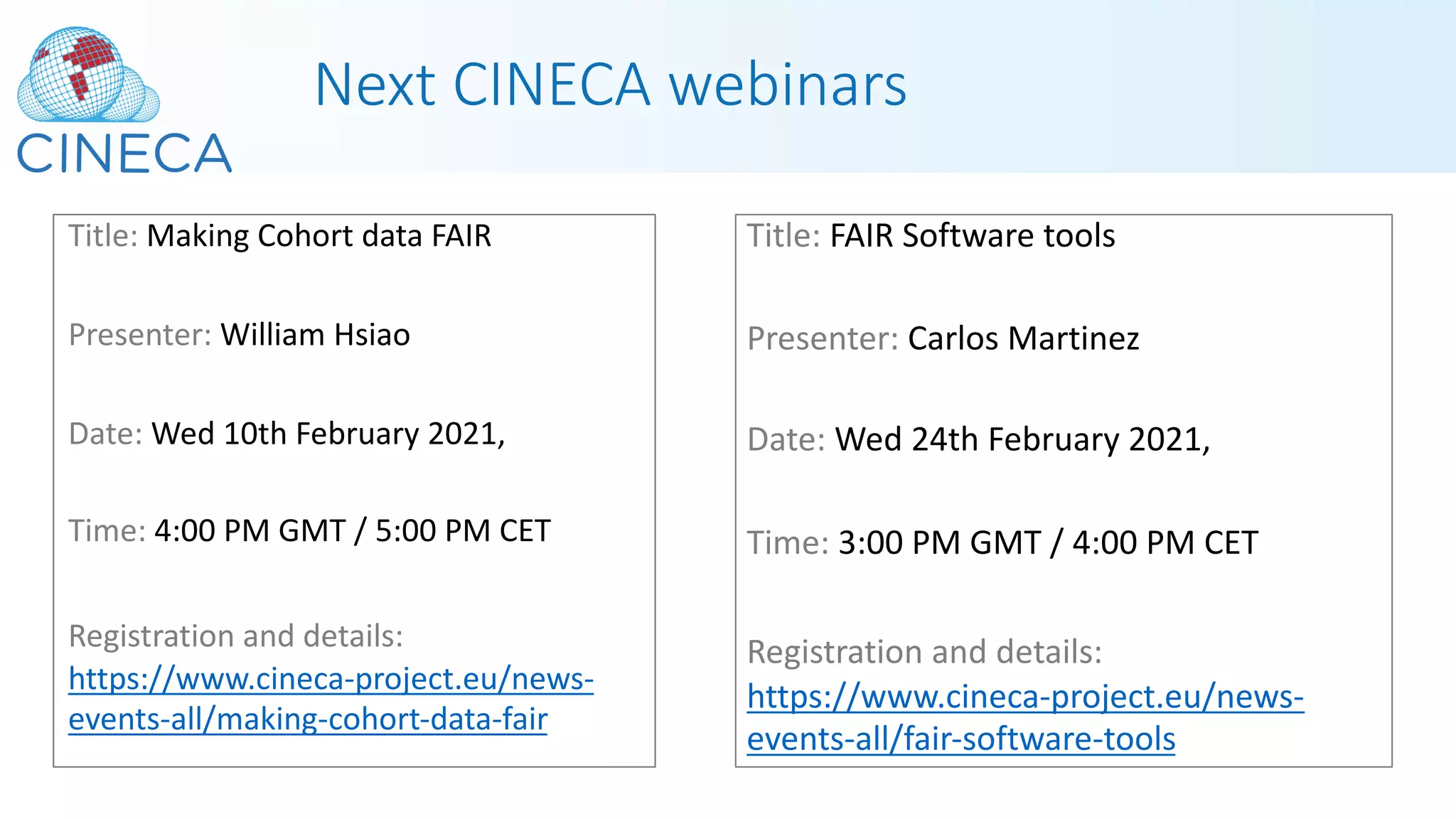
The document outlines a webinar series focusing on the FAIR principles in health data networks, particularly emphasizing their role in promoting open science and improving medical evidence generation. It discusses the challenges and advancements in sharing and standardizing health data, especially in light of the COVID-19 pandemic, which has accelerated open science efforts. Led by Kees van Bochove, the presenter highlights the necessity of equitable data access and the transformation of research practices in the biomedical field.