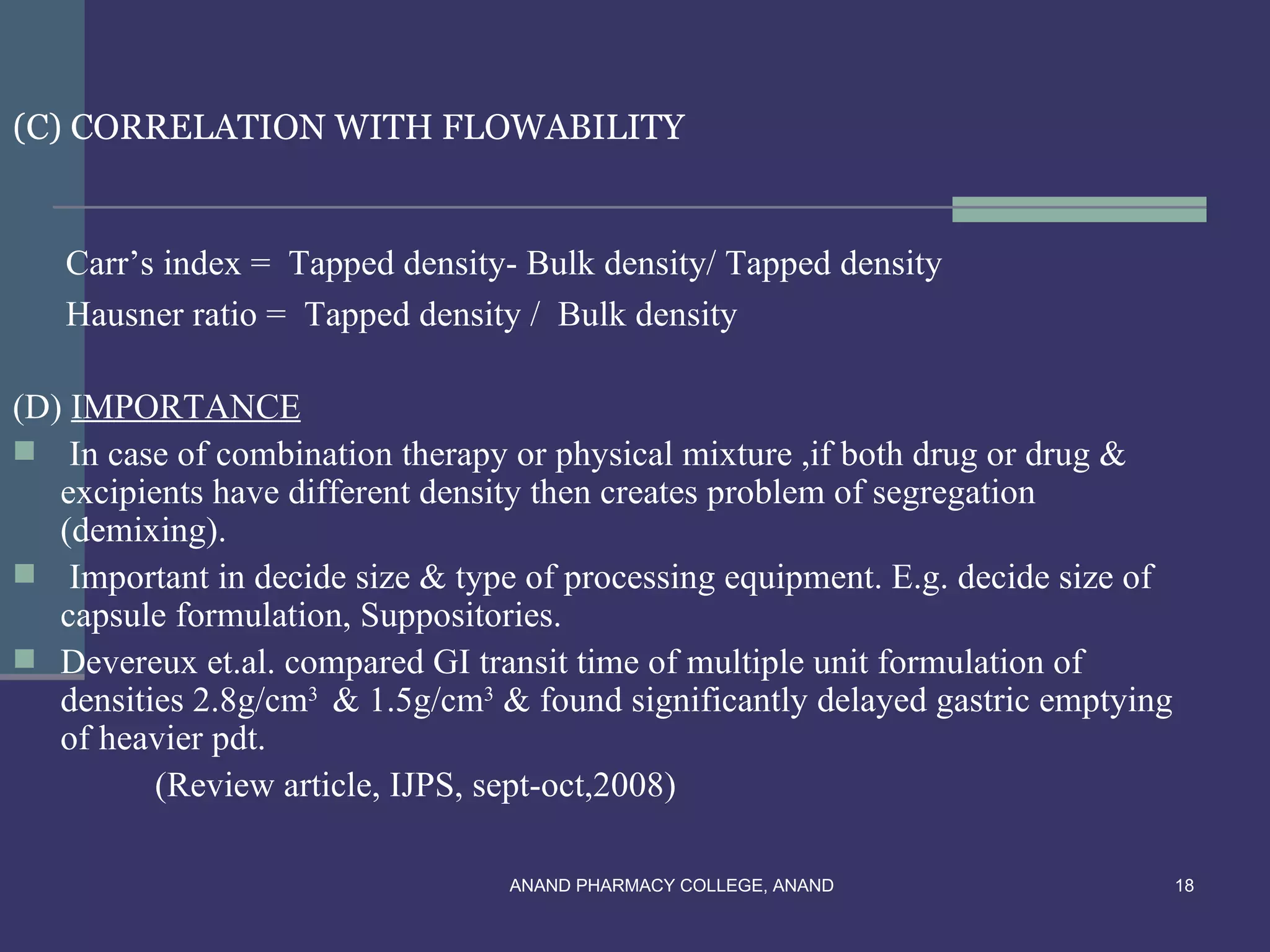
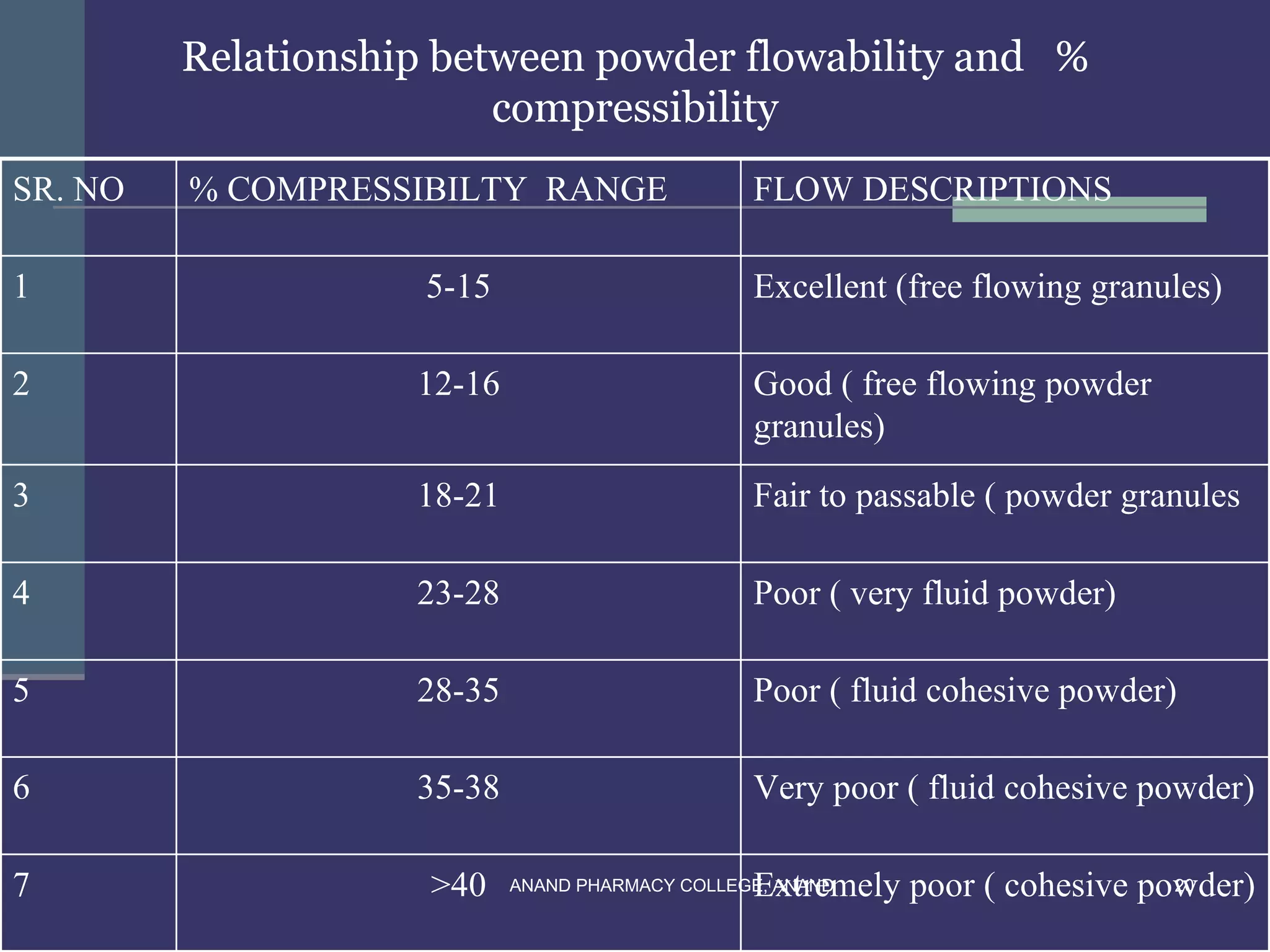
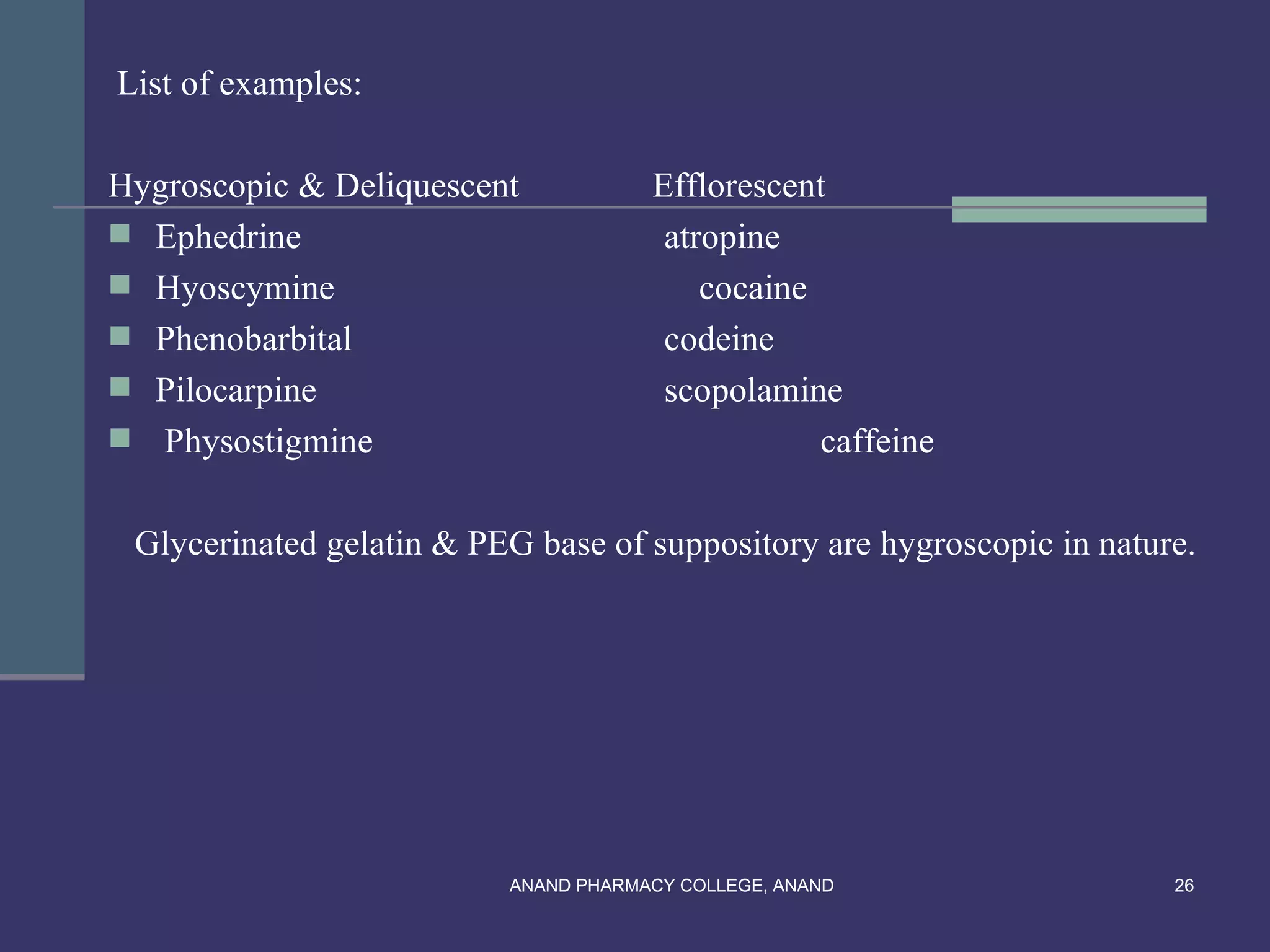
This document provides an overview of preformulation factors affecting dosage forms. It discusses properties like flow, density, compressibility, and others that influence the development of safe and effective drug dosage forms. The goal of preformulation is to design dosage forms with good bioavailability. Various methods for characterizing properties are described, along with their importance in determining the suitable dosage form for a drug.











![3. ELASTICITY
E.g. paracetamol, acetyl salicylic acid
If material is elastic, it rebound when compression force is released.
Elastic material may lead to capping & lamination
They require wet massing to induce plasticity or plastic tableting material.
4. PUNCH FILMING [STICKING]:
This may lead to chipping of tablet.
ANAND PHARMACY COLLEGE, ANAND 22](https://image.slidesharecdn.com/preformulation-120318030500-phpapp02/75/Preformulation-22-2048.jpg)














![D)IMPORTANCE
[1] FLUID
For mixing
For particle size reduction of disperse system
Passing though orifice, pouring, packaging in bottle,
passing though hypodermic needle.
Flow though pipe
Physical stability of disperse system
[2] QUASISOLIDS
Spreading and adherence to skin
Removal from jar
Capacity of solids to mix with liquid
Release of drug from base COLLEGE, ANAND
ANAND PHARMACY 44](https://image.slidesharecdn.com/preformulation-120318030500-phpapp02/75/Preformulation-44-2048.jpg)
![[3] SOLID
Flow of powder from hopper and into a die cavity in tableting or in
encapsulation
Packagability of powder or granules solids.
[4] PROCESSING
Production capacity of the equipment
Processing efficiency
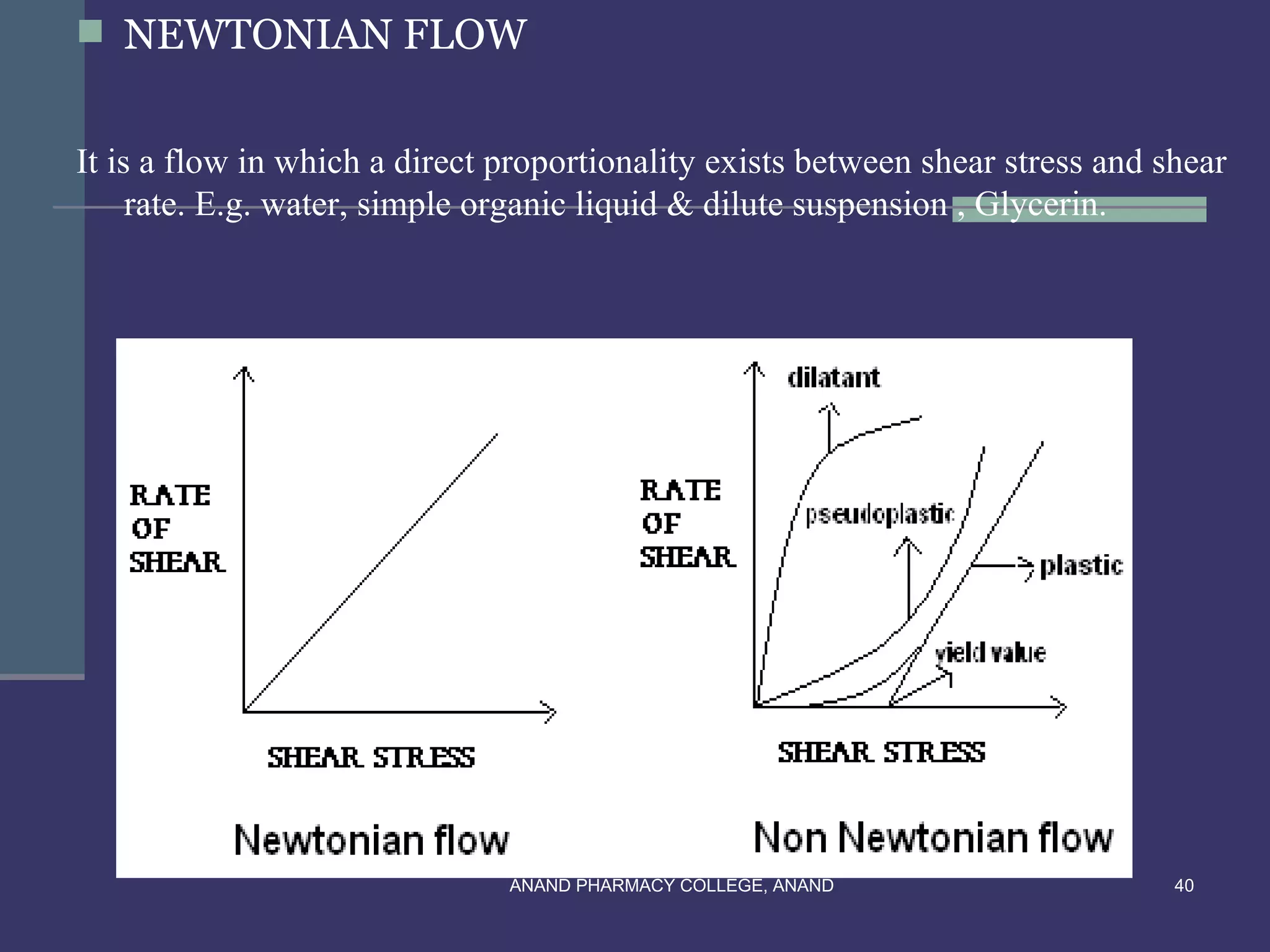
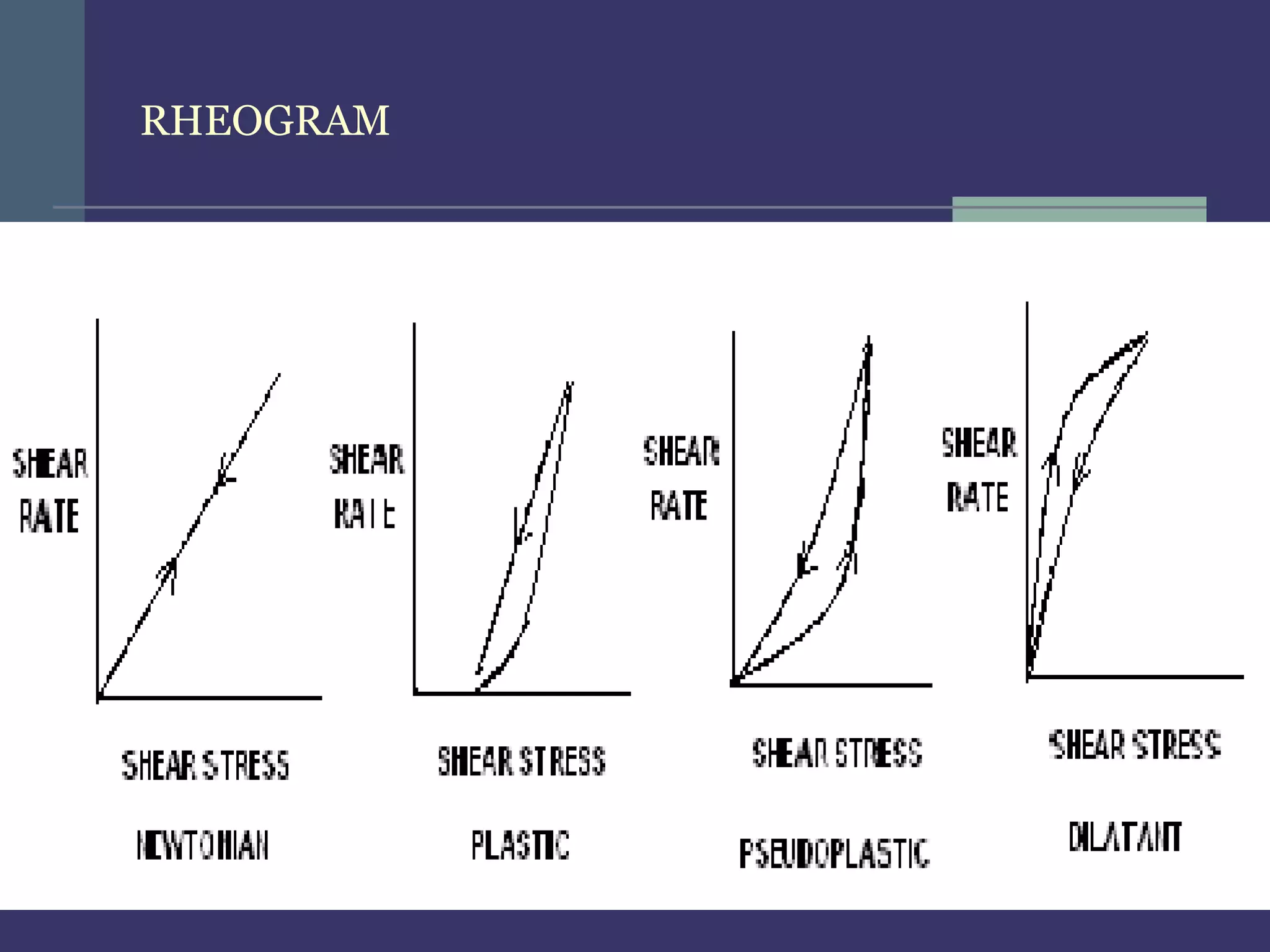
THIXOTROPHY:
In thixotropy apply shear stress convert gel – sol & remove shear stress convert
sol – gel, means gel to sol to gel.
Application :- for stability of suspension
e.g. conc. Parental suspension containing 40-70% w/v of procaine penicillin G
ANAND PHARMACY COLLEGE, ANAND 45](https://image.slidesharecdn.com/preformulation-120318030500-phpapp02/75/Preformulation-45-2048.jpg)





