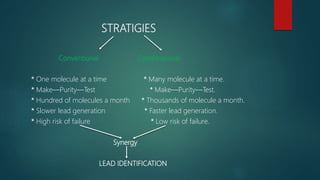
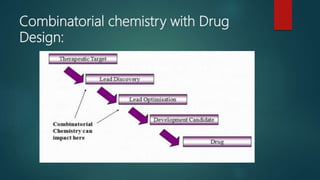
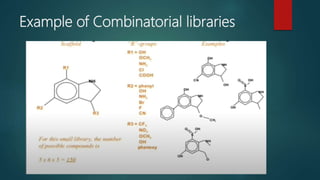
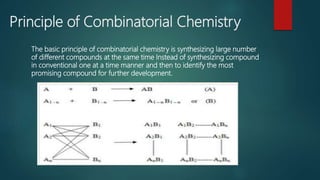
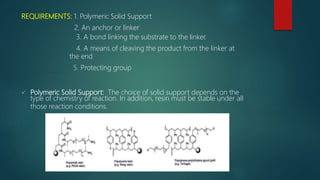
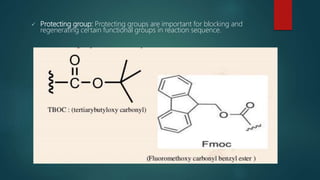
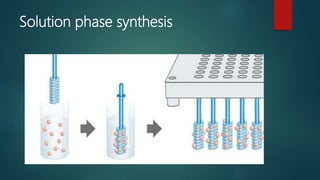
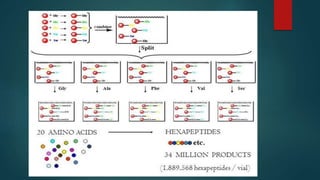
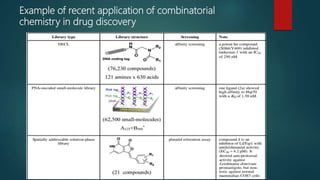
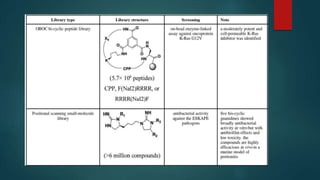
This document discusses combinatorial chemistry, which is a technique used to rapidly generate large libraries of compounds for screening and drug discovery. It defines combinatorial chemistry as producing large numbers of similar molecules using the same reaction conditions. The key principles are that it allows preparation of thousands of compounds per month using parallel synthesis techniques like solid and solution phase chemistry. This increases the chances of identifying hit compounds for pharmaceutical development compared to traditional synthetic methods. Applications of combinatorial chemistry include drug discovery, agrochemical and biotechnology research by creating molecular diversity libraries for high-throughput screening.