Amines fy b. pharmacy pci
•
20 likes•2,110 views
Aliphatic amines - Basicity, Effect of substituent on basicity, Qualitative test, Structure and uses of- ethanolamine, ethylenediamine
Report
Share
Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Phenols: methods of preparation, chemical reaction

Phenols: methods of preparation, chemical reaction
Classification, Nomenclature and structural isomerism of organic compound 

Classification, Nomenclature and structural isomerism of organic compound
Similar to Amines fy b. pharmacy pci
Similar to Amines fy b. pharmacy pci (20)
class 12 chemistry amines formula and structure pdf

class 12 chemistry amines formula and structure pdf
Amines, Nomenclature, Physical properties and Chemical by Shabab

Amines, Nomenclature, Physical properties and Chemical by Shabab
More from AtulBendale2
More from AtulBendale2 (15)
2. furan Heterocyclic compounds short notes for Pharmacy students

2. furan Heterocyclic compounds short notes for Pharmacy students
4. pyrazole Heterocyclic compounds short notes for Pharmacy students

4. pyrazole Heterocyclic compounds short notes for Pharmacy students
5. imidazole Heterocyclic compounds short notes for Pharmacy students

5. imidazole Heterocyclic compounds short notes for Pharmacy students
6. oxazole Heterocyclic compounds short notes for Pharmacy students

6. oxazole Heterocyclic compounds short notes for Pharmacy students
11. acridine Heterocyclic compounds short notes for Pharmacy students

11. acridine Heterocyclic compounds short notes for Pharmacy students
Stereoselective and stereospecific reaction SEM IV POC III

Stereoselective and stereospecific reaction SEM IV POC III
Recently uploaded
Mehran University Newsletter is a Quarterly Publication from Public Relations OfficeMehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Recently uploaded (20)
HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx

HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx
21st_Century_Skills_Framework_Final_Presentation_2.pptx

21st_Century_Skills_Framework_Final_Presentation_2.pptx
ICT role in 21st century education and it's challenges.

ICT role in 21st century education and it's challenges.
Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...

Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
Kodo Millet PPT made by Ghanshyam bairwa college of Agriculture kumher bhara...

Kodo Millet PPT made by Ghanshyam bairwa college of Agriculture kumher bhara...
Exploring_the_Narrative_Style_of_Amitav_Ghoshs_Gun_Island.pptx

Exploring_the_Narrative_Style_of_Amitav_Ghoshs_Gun_Island.pptx
Amines fy b. pharmacy pci
- 1. A M I N E
- 2. At the end of session students should able to- 1 2. Explain basic nature of amines 3. Compare basicity of substituted amines 4. Explain functional group test for amine 5. Draw structure and list out various uses of amines
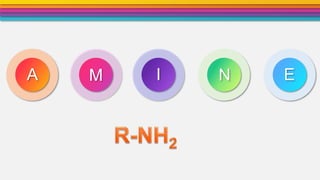
- 3. Definition2 Amines are the derivative of ammonia (NH3) in which one or more hydrogen atom are replaced by alkyl groups. •General formula R-NH2 or Ar-NH2 •(Where R= Alkyl group and Ar= Aromatic ring) NH2 NH diphenylamineAniline H CH3 H3C NH3C N H3C N CH3 trimethyl amine H Methyl amine H dimethyl amine CH3
- 4. Classification3 Amines are classified as primary (1⁰), secondary (2⁰) and tertiary (3⁰) according to the number of alkyl group attached to the nitrogen CH3H CH3 H3C NH3C N H3C N CH3 tertiary H primary H secondary
- 5. Basicity of amines Amines are basic in nature. • The presence of lone pair of electron on nitrogen atom makes it basic. This lone pair of electron is radially available for the formation new N-H bond. • The substance which donate electron in reaction is a base according to Lewis concept. 1 HH + + - + R N H ClR N HCl H Salt H Amine Base acid
- 6. Relative basicity of alkyl amines2 • Here as the substitution of alkyl group increases basicity increases • More alkyl group more rapid the electron donation process. • More stable new N-H bond and positive charge on nitrogen.
- 7. Electron donating group e.g. alkyl group increases the basicity Electron withdrawing group e.g. Phenyl ring decreases the basicity 3
- 8. Aniline4 Aniline is stabilized by Resonance that is delocalization of electron as shown below: NH2
- 9. Basicity of aniline with substituents5 • This is because, in 2,4,6- trinitro aniline internal hydrogen bonding permits delocalization of lone pair of electron on N hence less available for new N-H bond (Less basic) • In 2,4,6- trinitro-N,N-dimethyl aniline due to strong steric inhibition of resonance, lone pair of electron on N is radially available for new N- H bond (More basic) H HH3C CH3 O N ON -++ ONN- O NO2O2N + NNO2 - OO 2,4,6-trinitro aniline 2,4,6-trinitro N,N-dimethyl aniline 2,4,6- trinitro-N,N-dimethyl aniline is more basic than 2,4,6- trinitro aniline
- 10. Qualitative test: Amine Nitrous Acid test: 1 Test Observation Inference 0.2 g or 0.2 ml substance + 2 ml conc. HCl + 2 ml water and boil. Cool in ice bath to 5⁰C. Add 2 ml NaNO2 solution and mix. 1 Evolution of N2 gas as bubble 2. Yellow oily layer 3. No visible sign of reaction i.e. observation 1 & 2 absent 1. Primary amine 2. Secondary amine 3. Tertiary amine ++ Mixture of productHNO2 N2R NH2 Bubble primary amine
- 11. Nitrous acid test reaction2
- 12. Hinsberg test 3 2. Secondary 3. Tertiary amine Test Observation Inference 0.2 g or 0.2 ml substance + 2 ml Pyridine + 2 ml Fresh 2% NaOH. Shake well and add 2 drops of benzene sulphonyl chloride. 1. ppt soluble in NaOH 2. ppt insoluble in NaOH 3. No ppt 1. Primary amine amine
- 14. Qualitative test: Amine5 Carbyl amine test: Test Observation Inference 0.2 g or 0.2 ml substance + 2 ml alcoholic KOH + 2-3 drop chloroform and warm carefully. Evil smell Primary amine Secondary & Tertiary amine do not give this reaction This test is used to distinguish primary amine from secondary and tertiary amine ++ ++ 3H O + 3KClCHCHCl3 R N3KOH 2 R NH2 Isocynate (Evil Smell) primary amine
- 15. Qualitative test: Amine Azo dye test 6 present Test Observation Inference Test tube 1- 0.2 g or 0.2 ml substance + 2 ml dil. HCl & cool in ice bath to 5⁰C. Test tube 2- 2 ml NaNO2 solution & cool in ice bath to 5⁰C and mix test tube 1 & 2. To this add ice cooled solution of β-napthol in NaOH. Formation of orange red dye stuff Primary aromatic amine is
- 16. Azo dye test reaction7
- 17. IUPAC: 2-Aminoethanol 1 For the preparation of buffer solution In production of detergent As emulsifier As corrosion inhibitor In making of hair dye/colour H H H2N C C OH H H
- 18. Structure and Uses : Ethylenediamine2 IUPAC: Ethane-1,2-diamine To produce chelating agent EDTA (Ethylenediamine tetra acetic acid) Used for making intermediate drug / chemical In manufacturing of surfactant and dyes Widely used precursor to various polymer Used in binder, adhesive H H H2N C C NH2 H H
- 19. Structure and Uses: Amphetamine3 IUPAC: 1-phenylpropan-2-amine To treat nacrolepsy i.e. sleep disorder To treat obesity To improve physical (athletic) performance i.e. to increase muscle strength For enhancing cognitive performance i.e. memory Side effect- insomnia, fatigue, mood swing H H C C NH2 H CH3