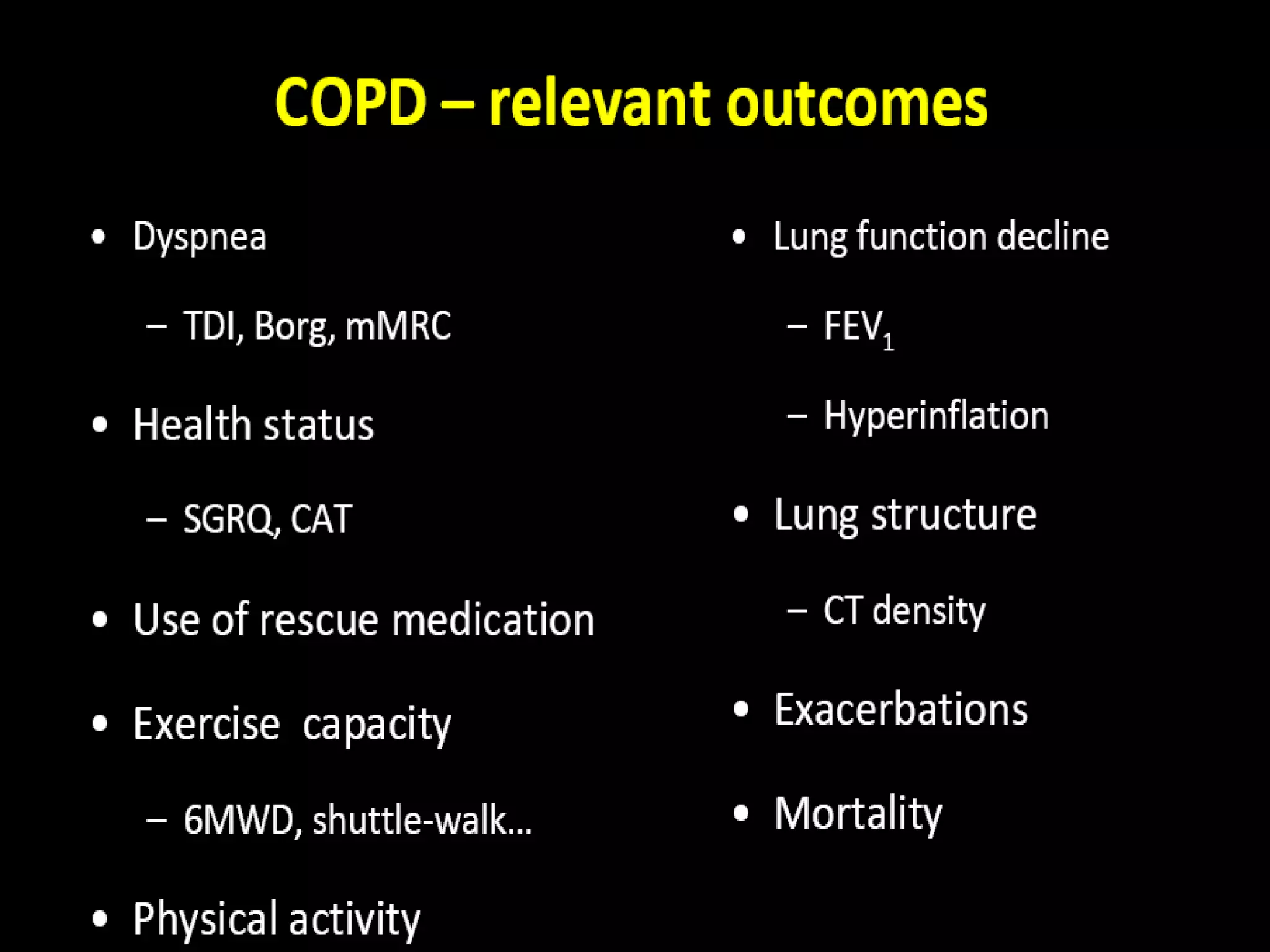
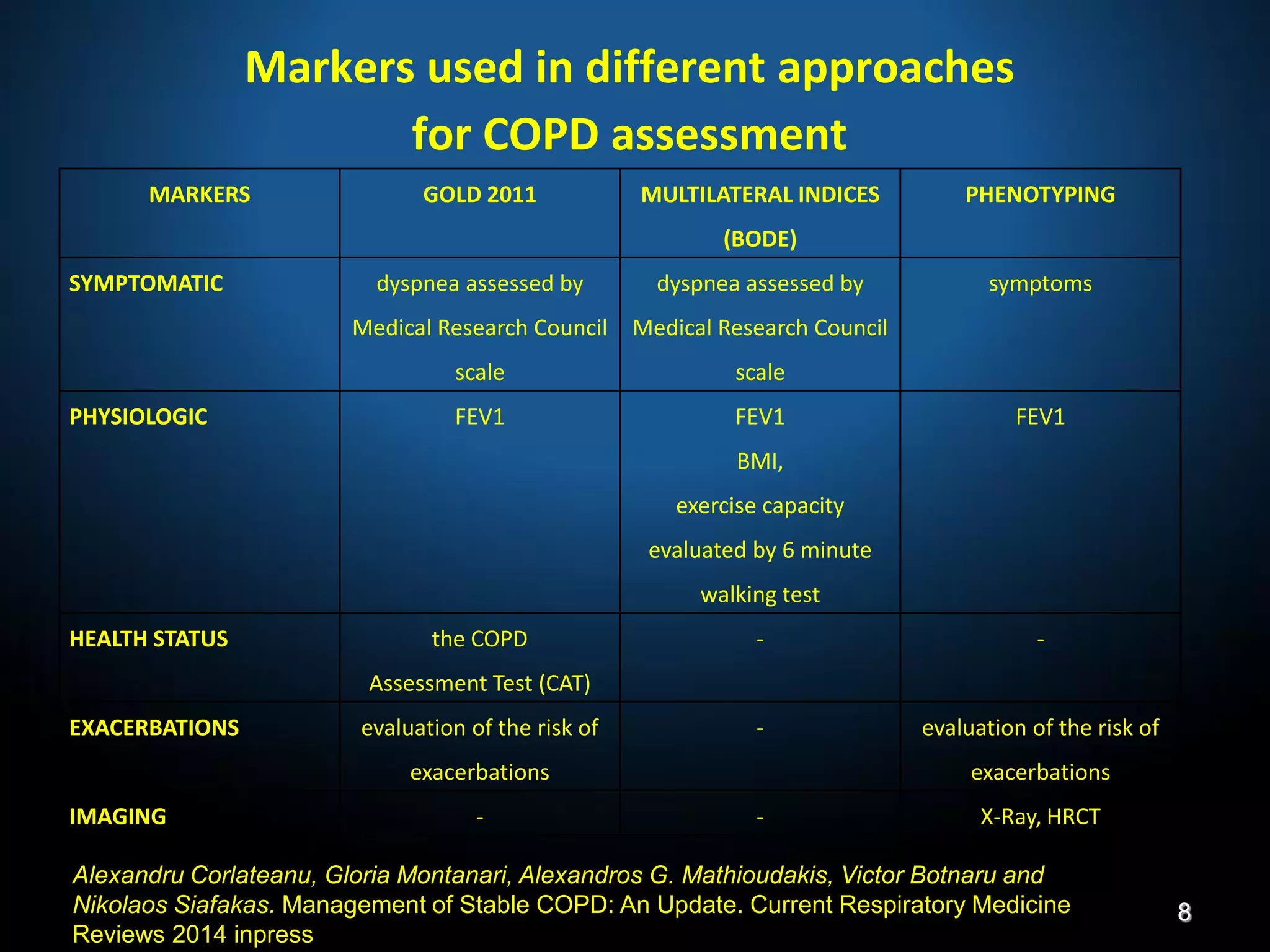
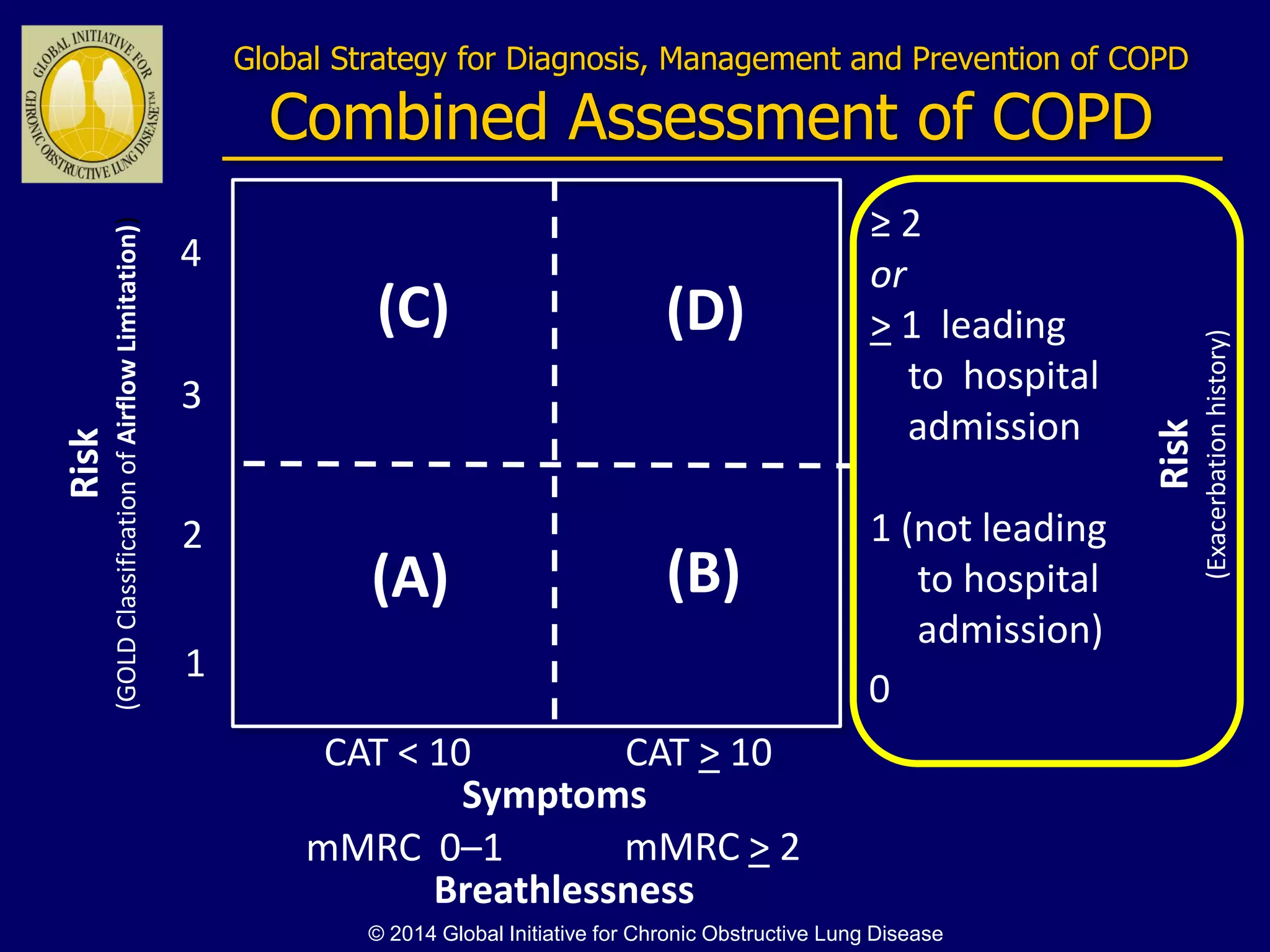
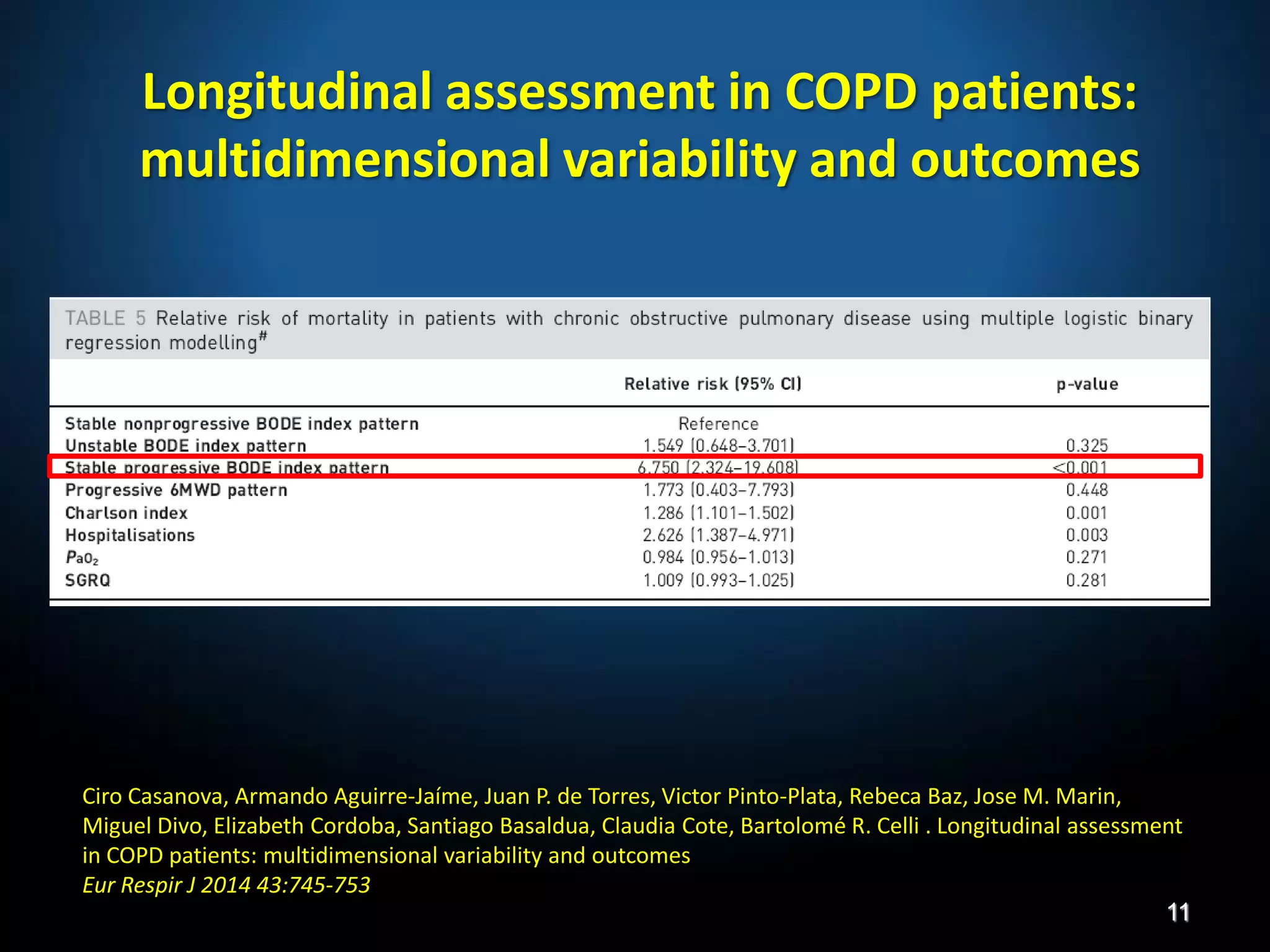
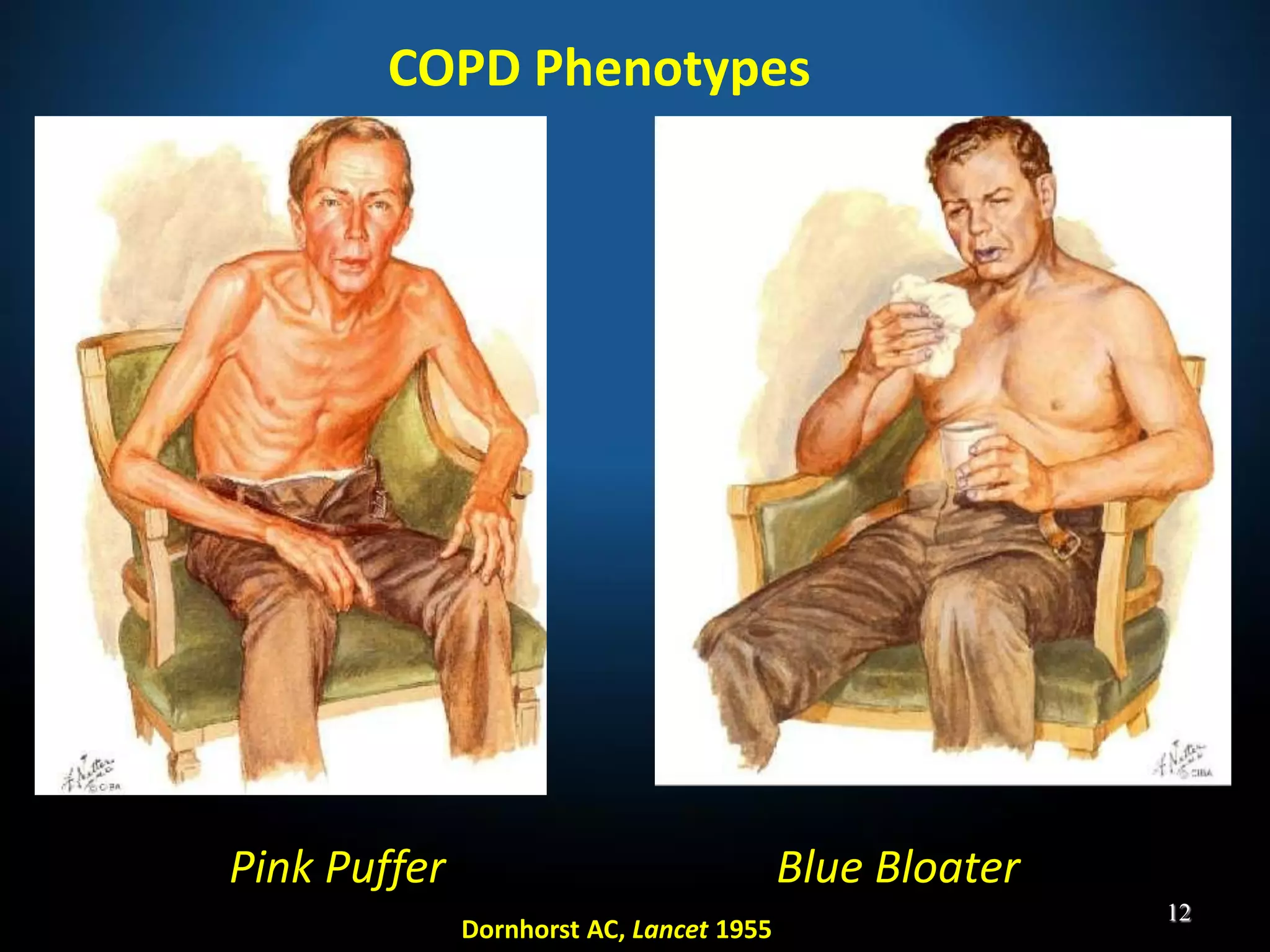
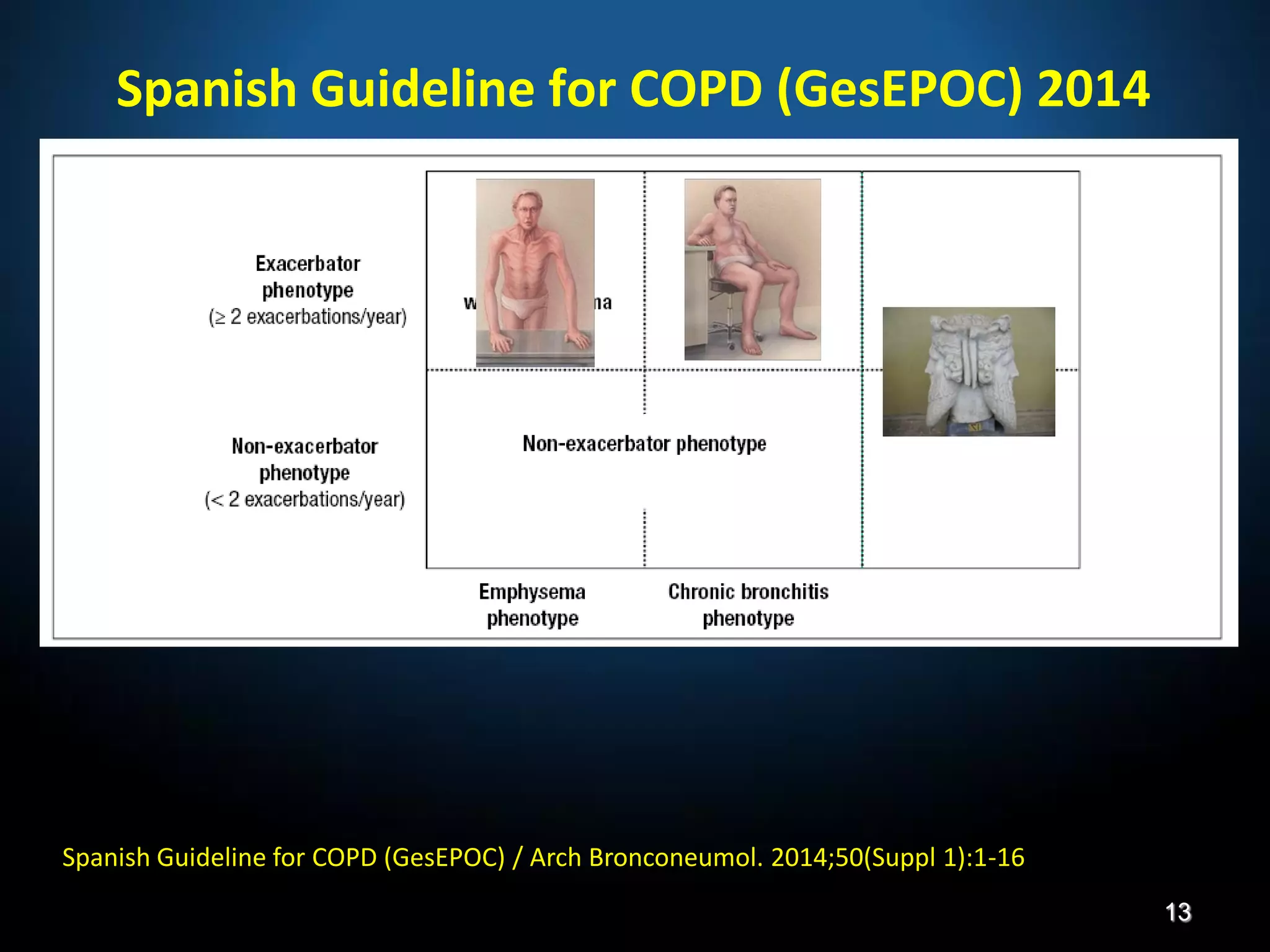
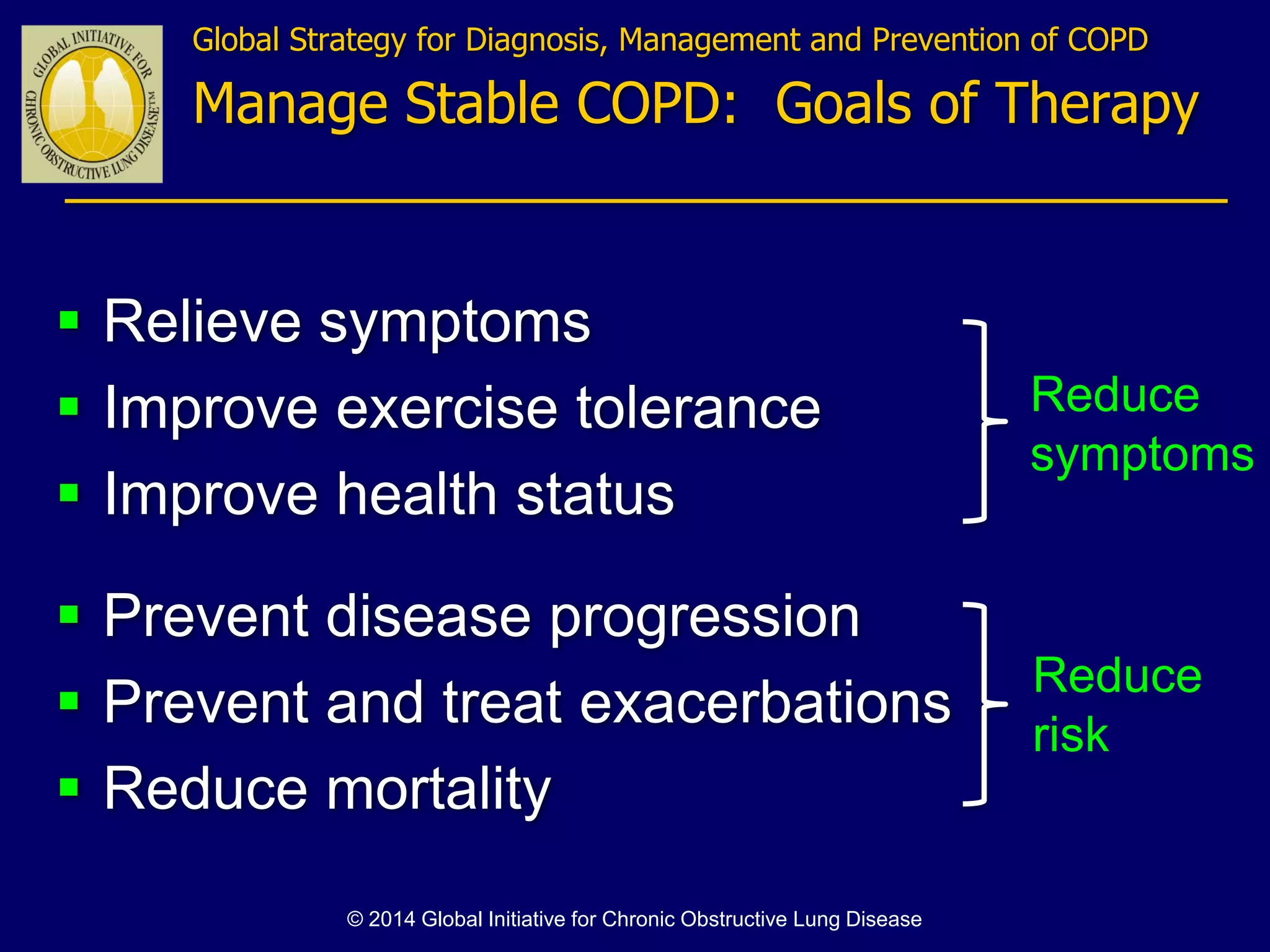
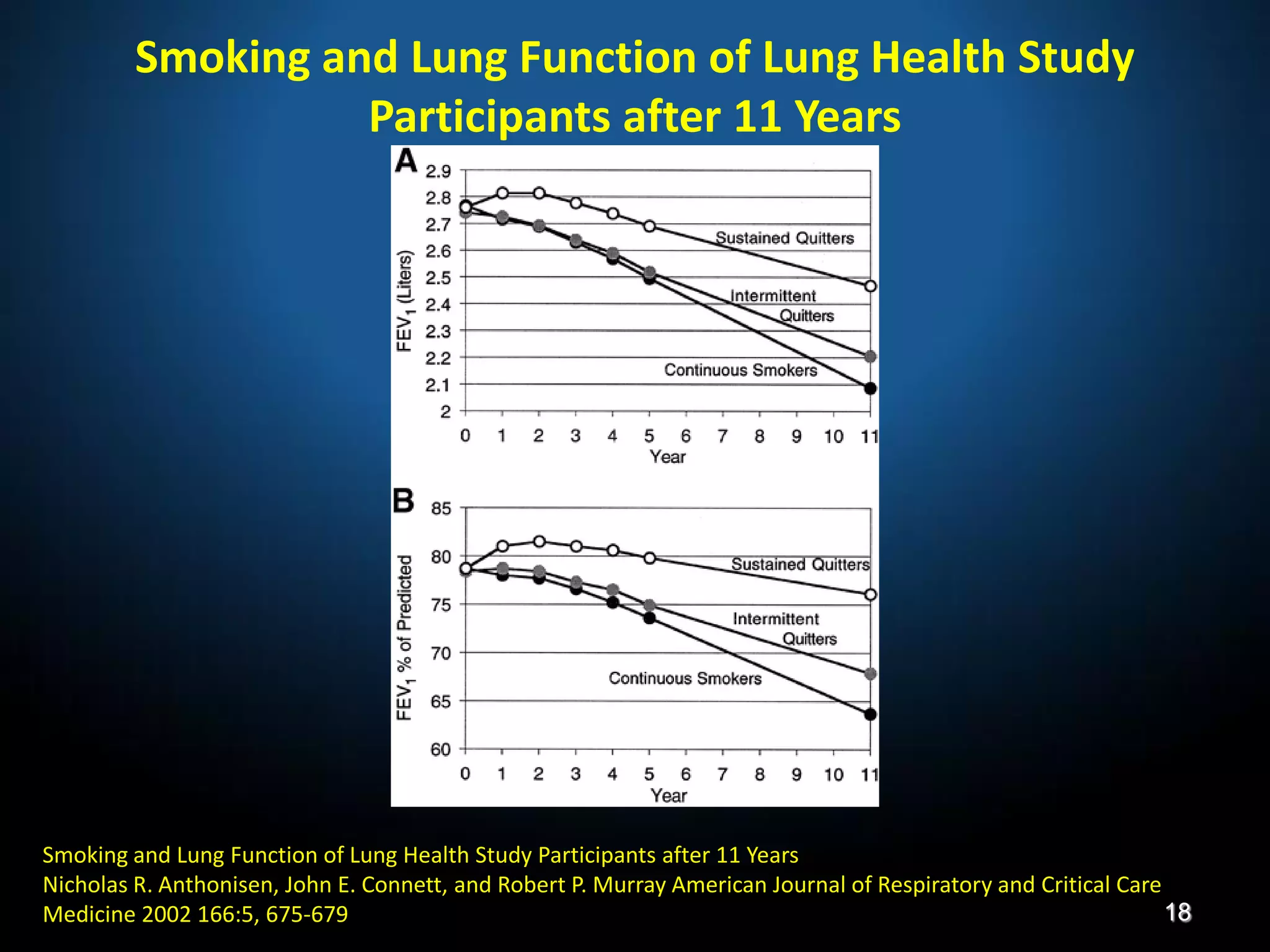
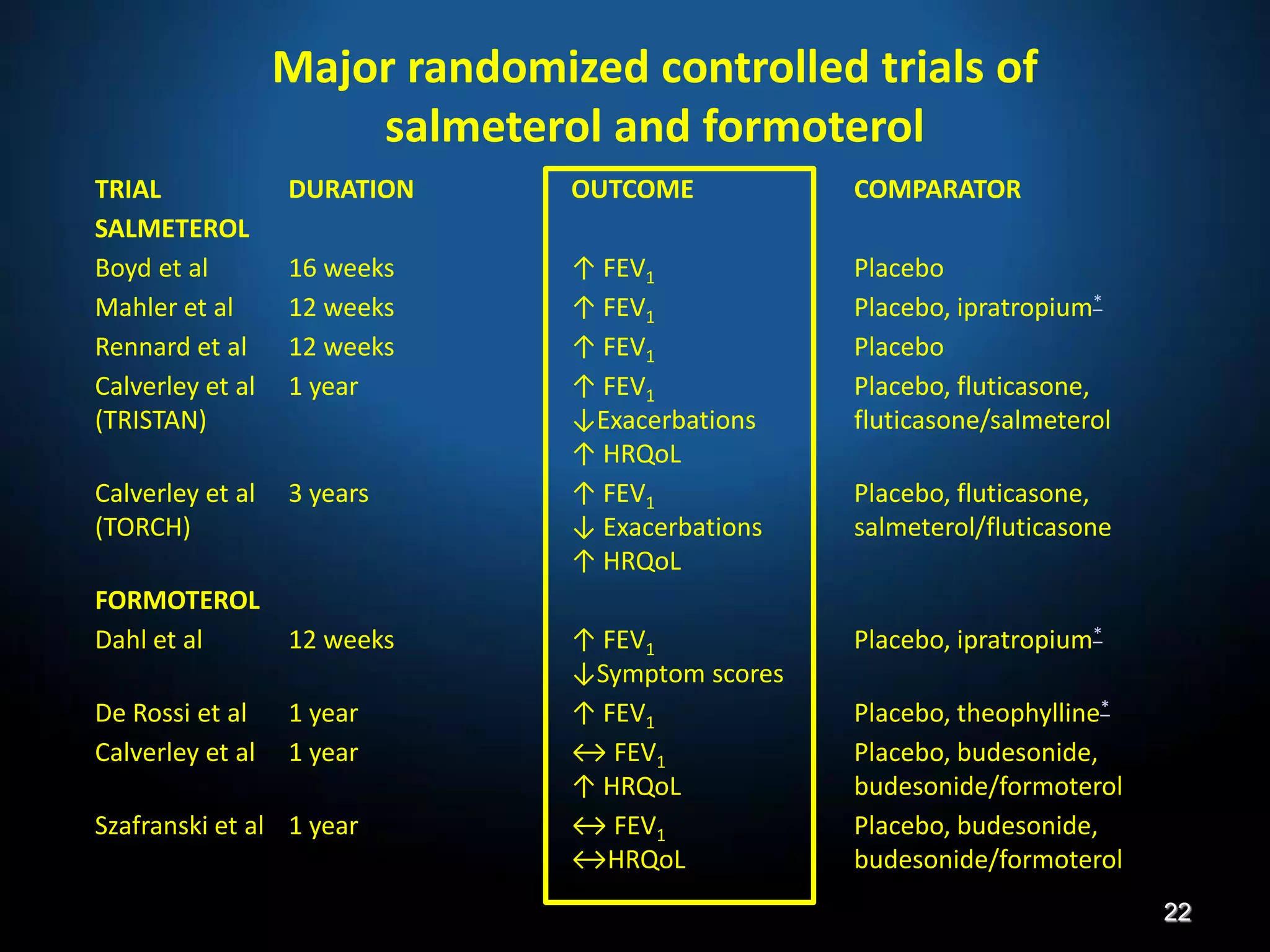
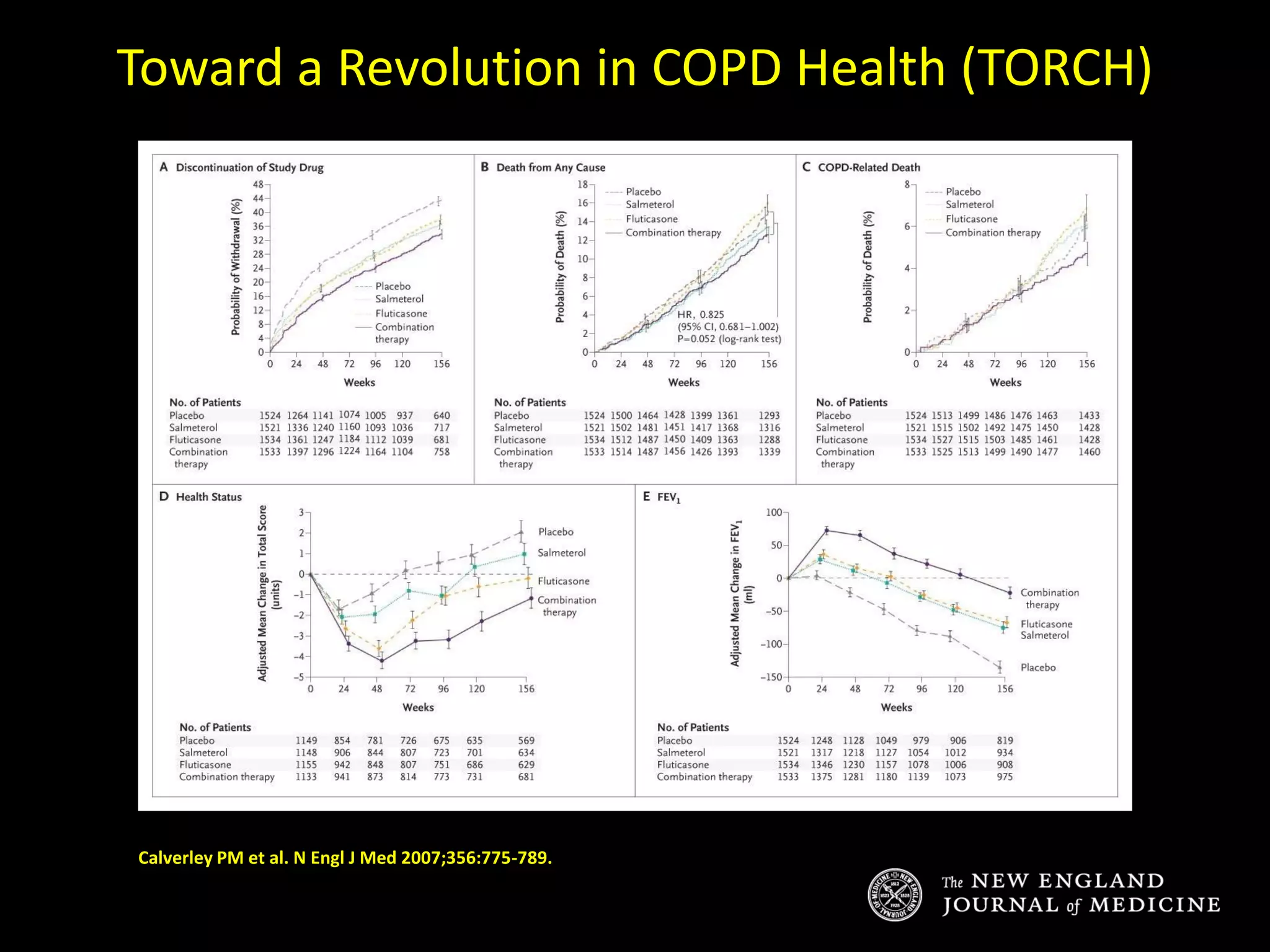
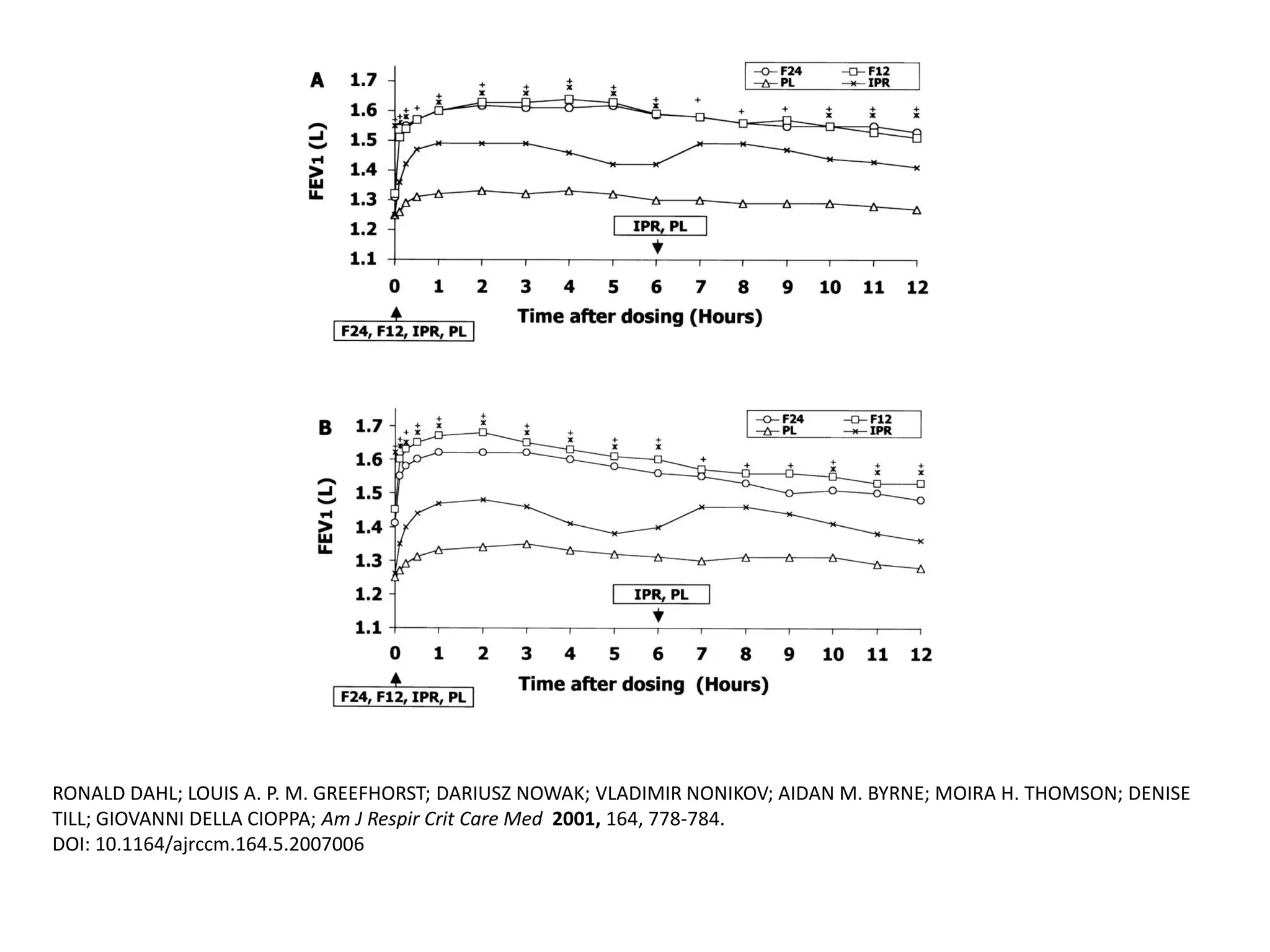
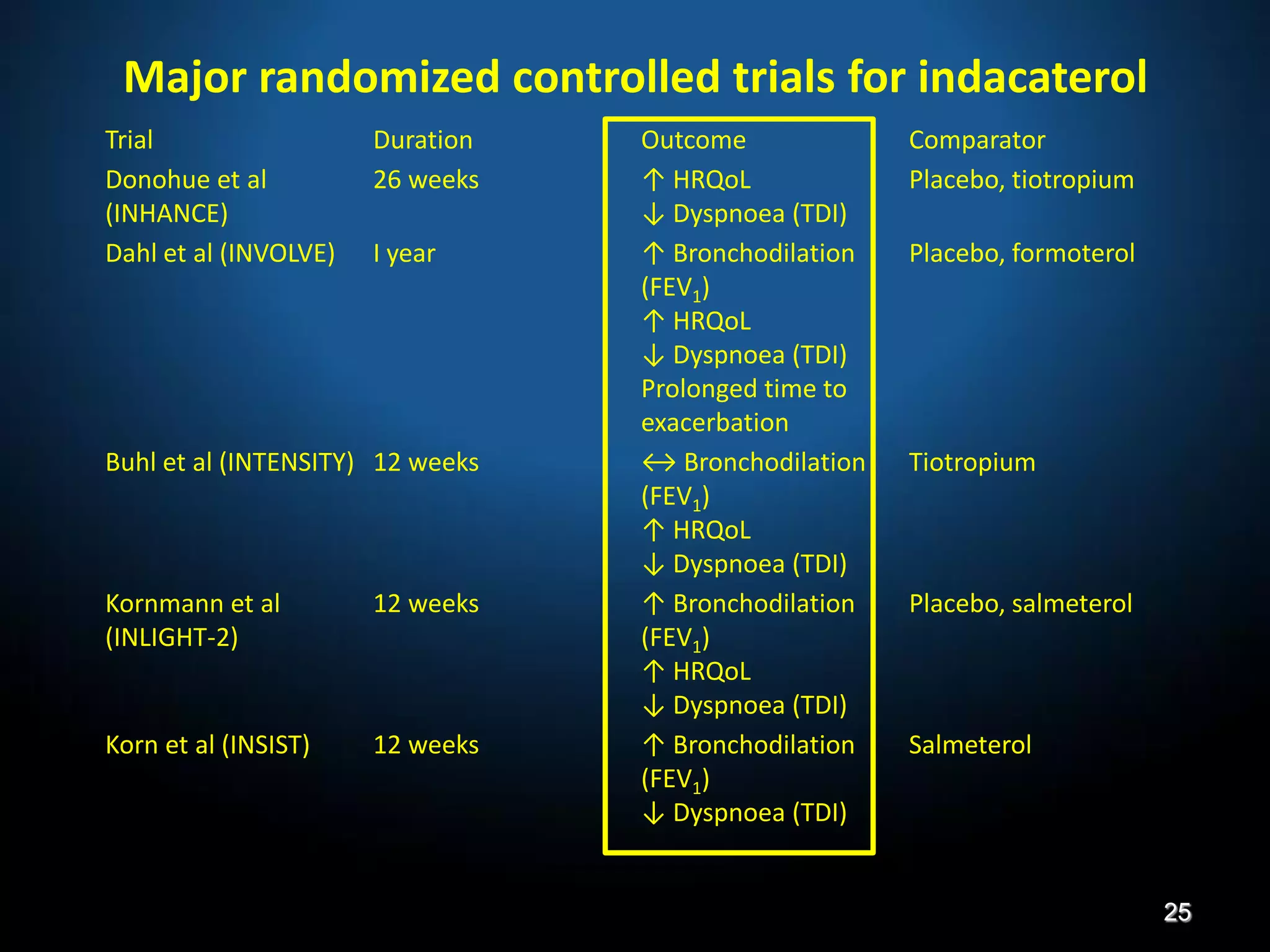
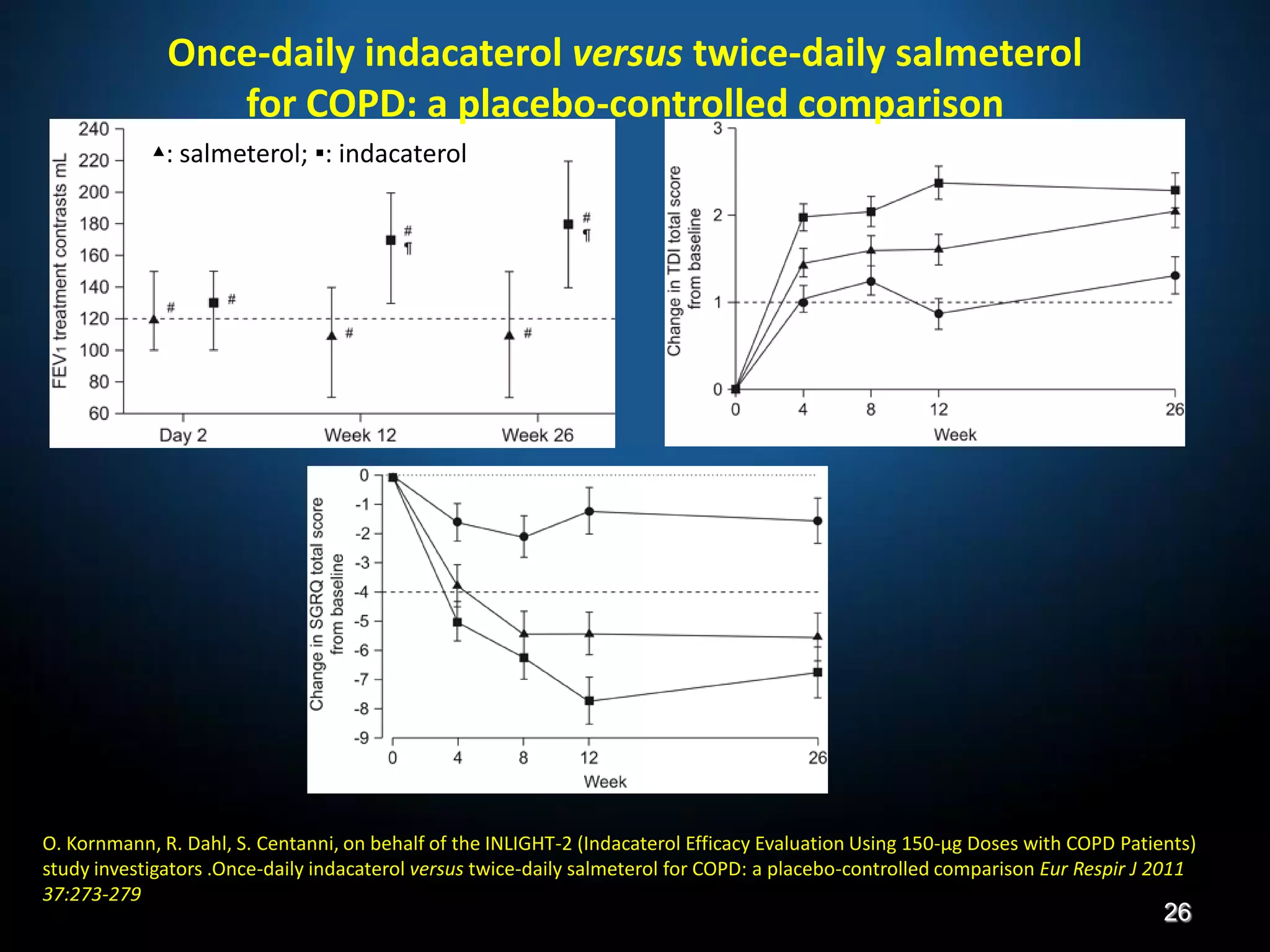
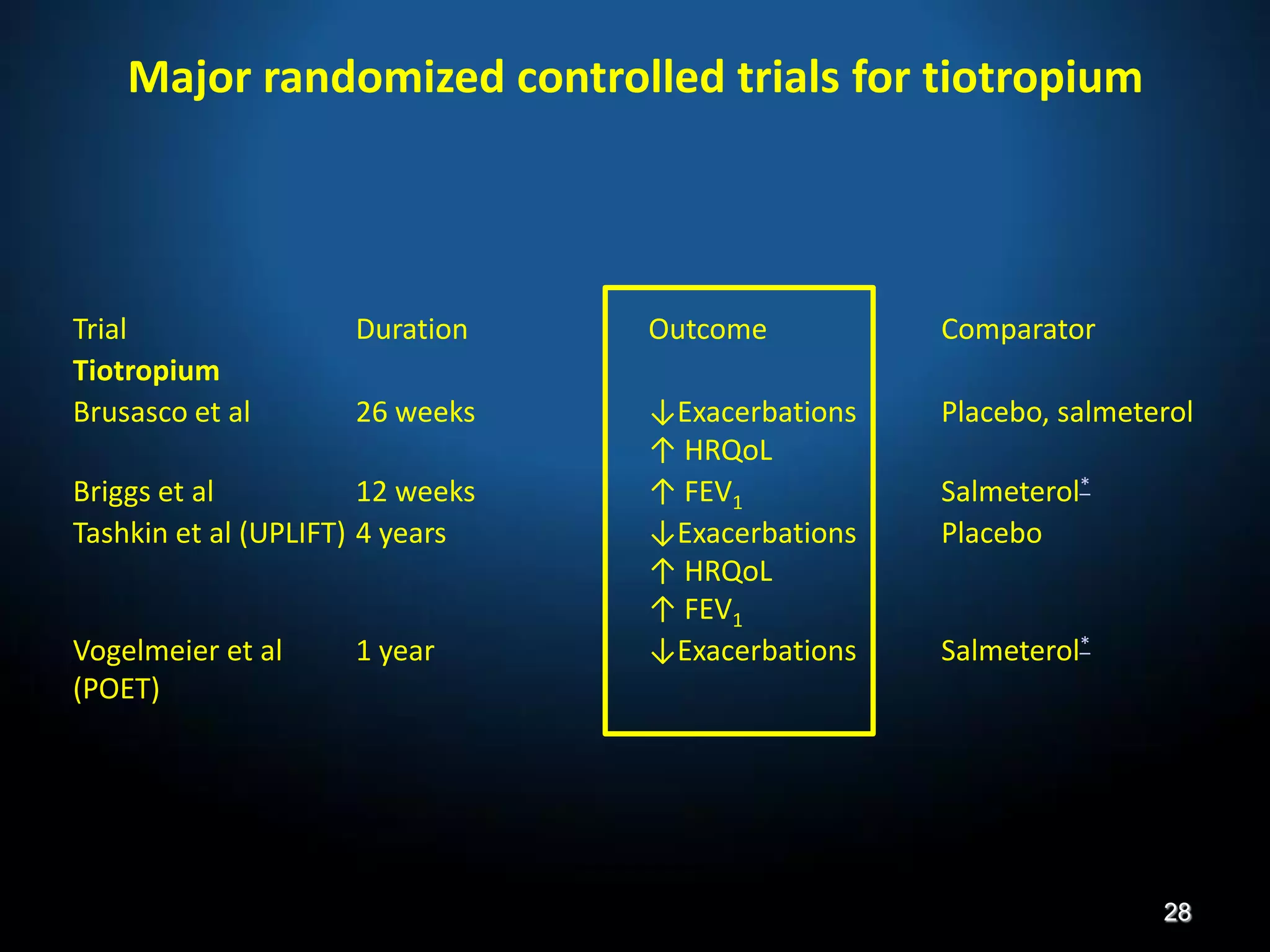
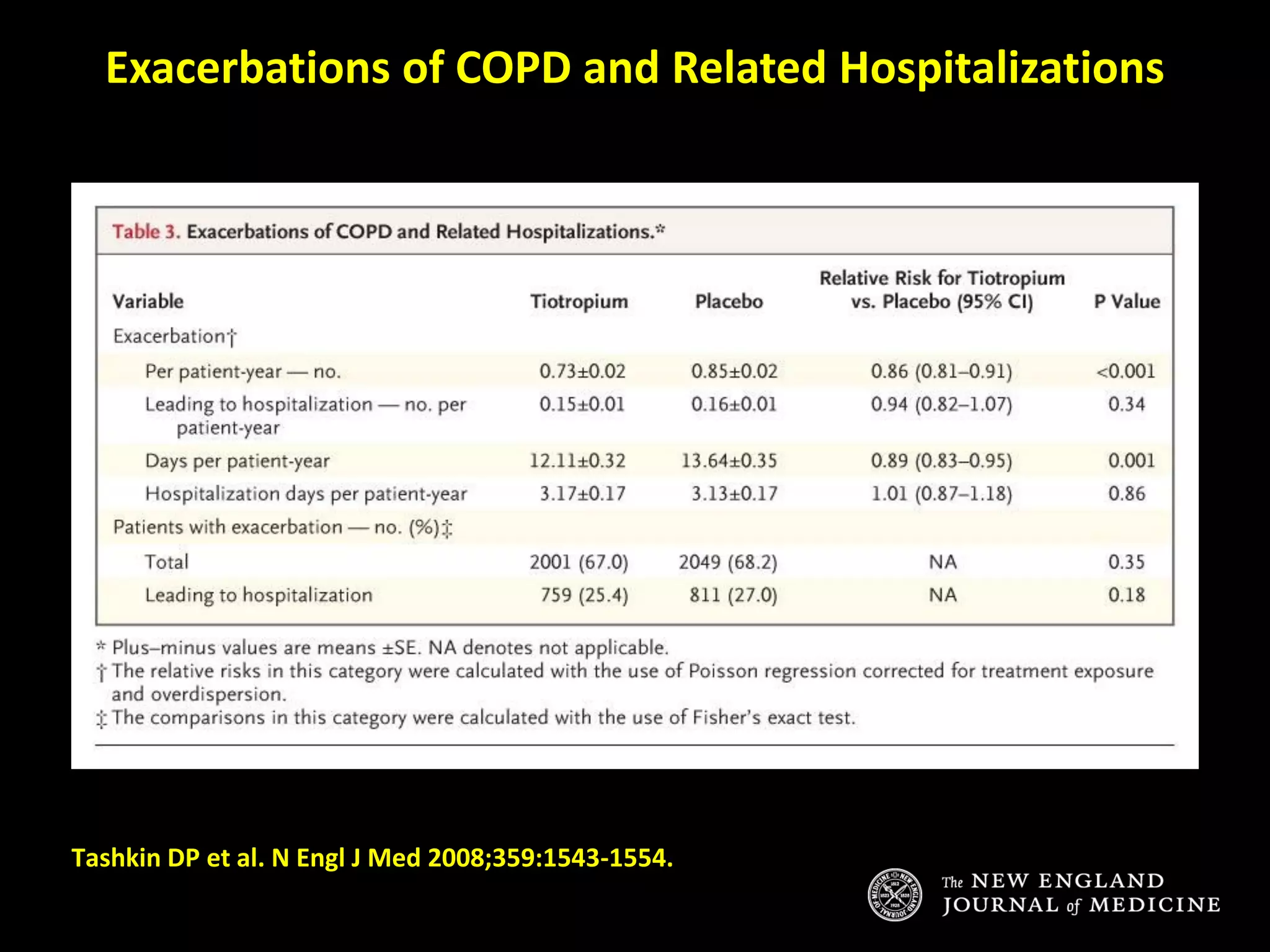
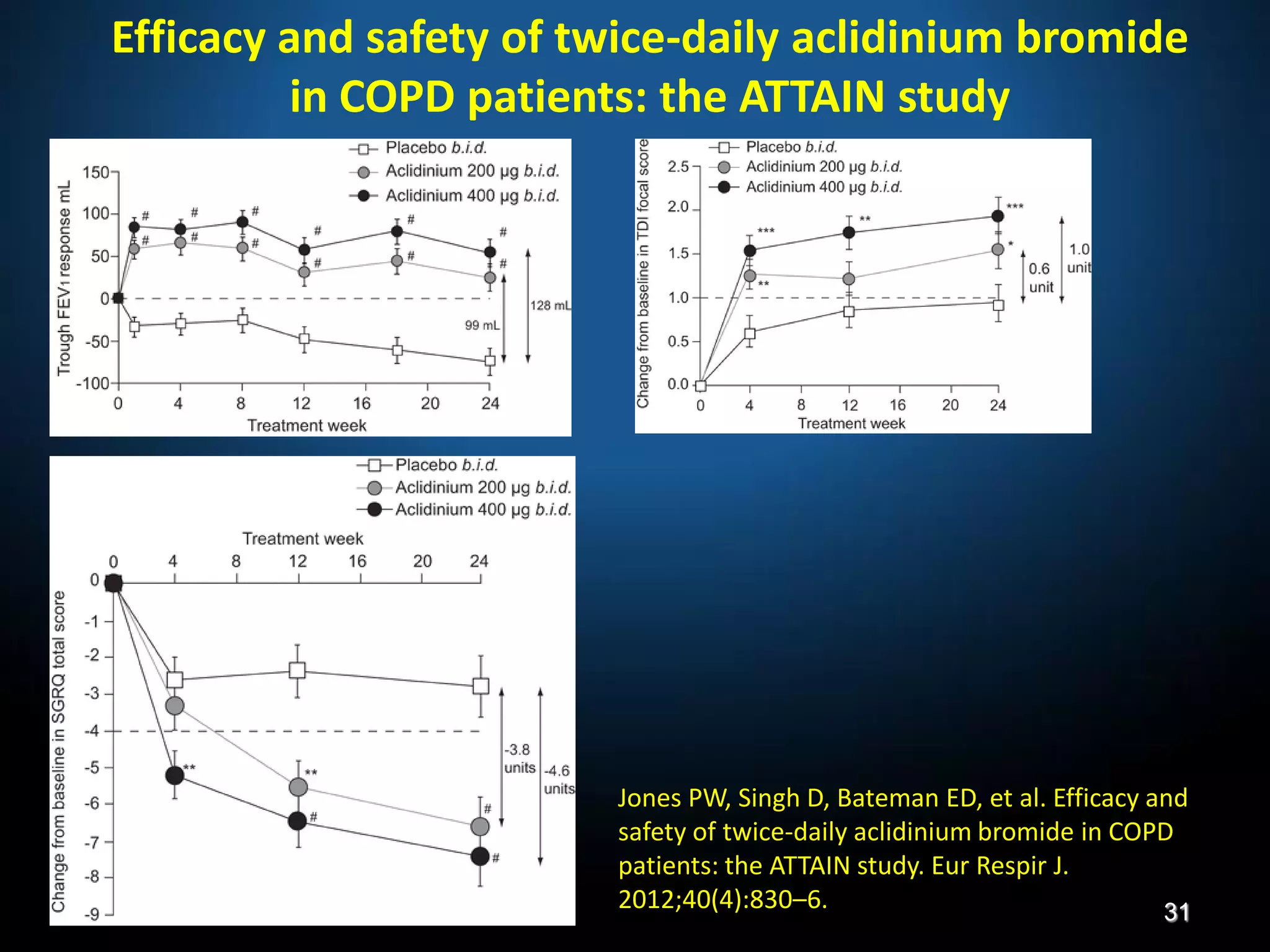
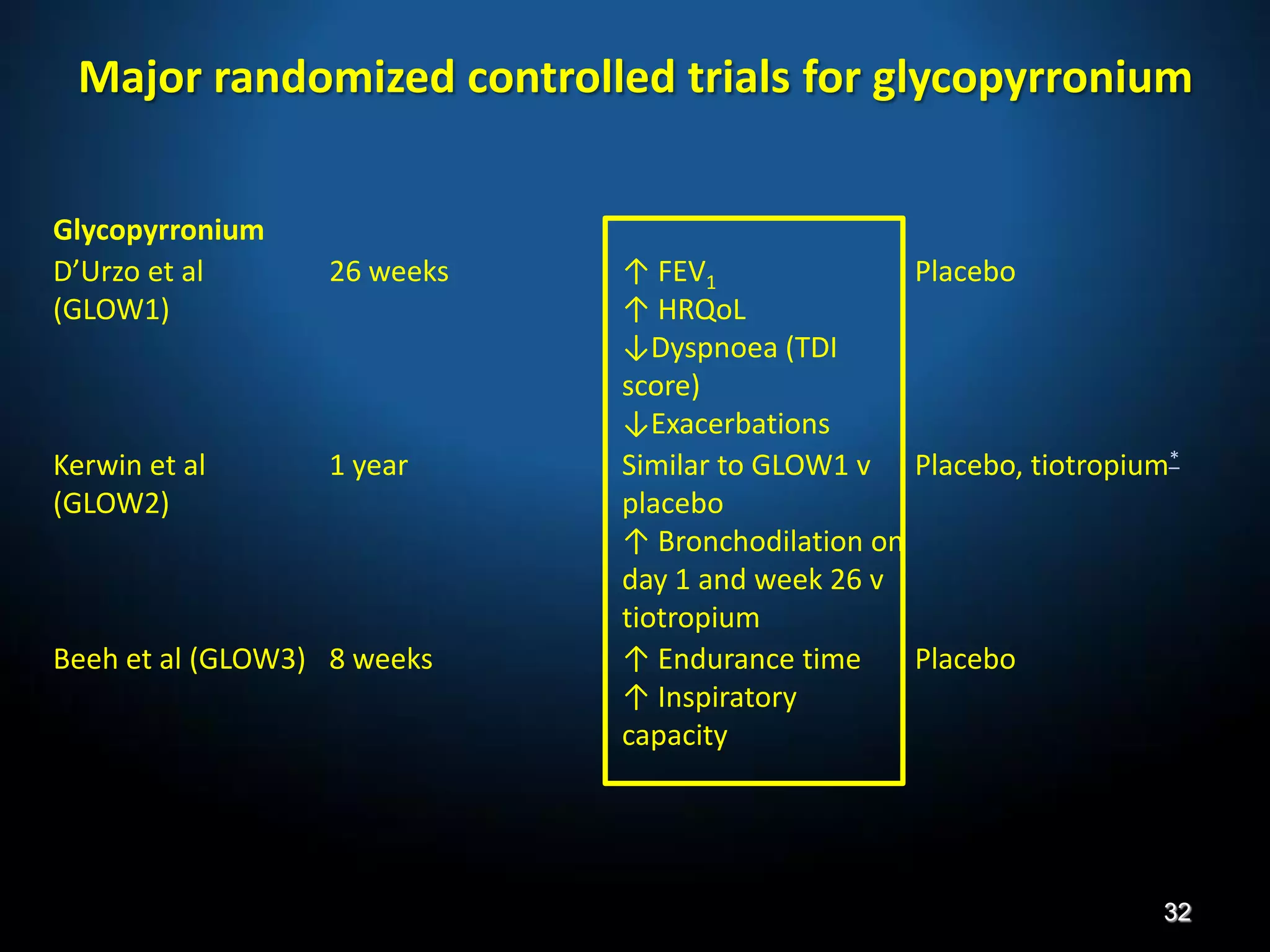
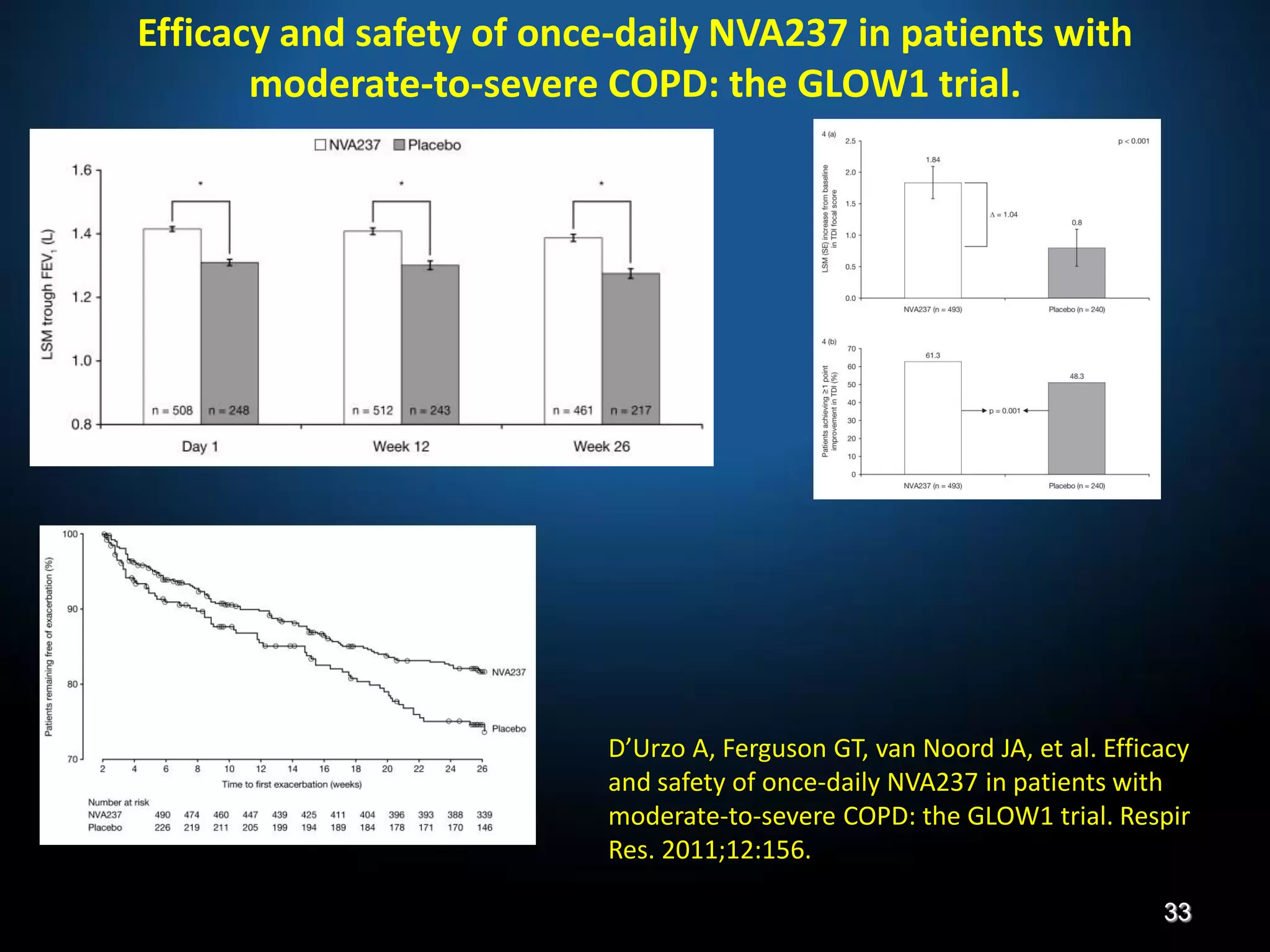
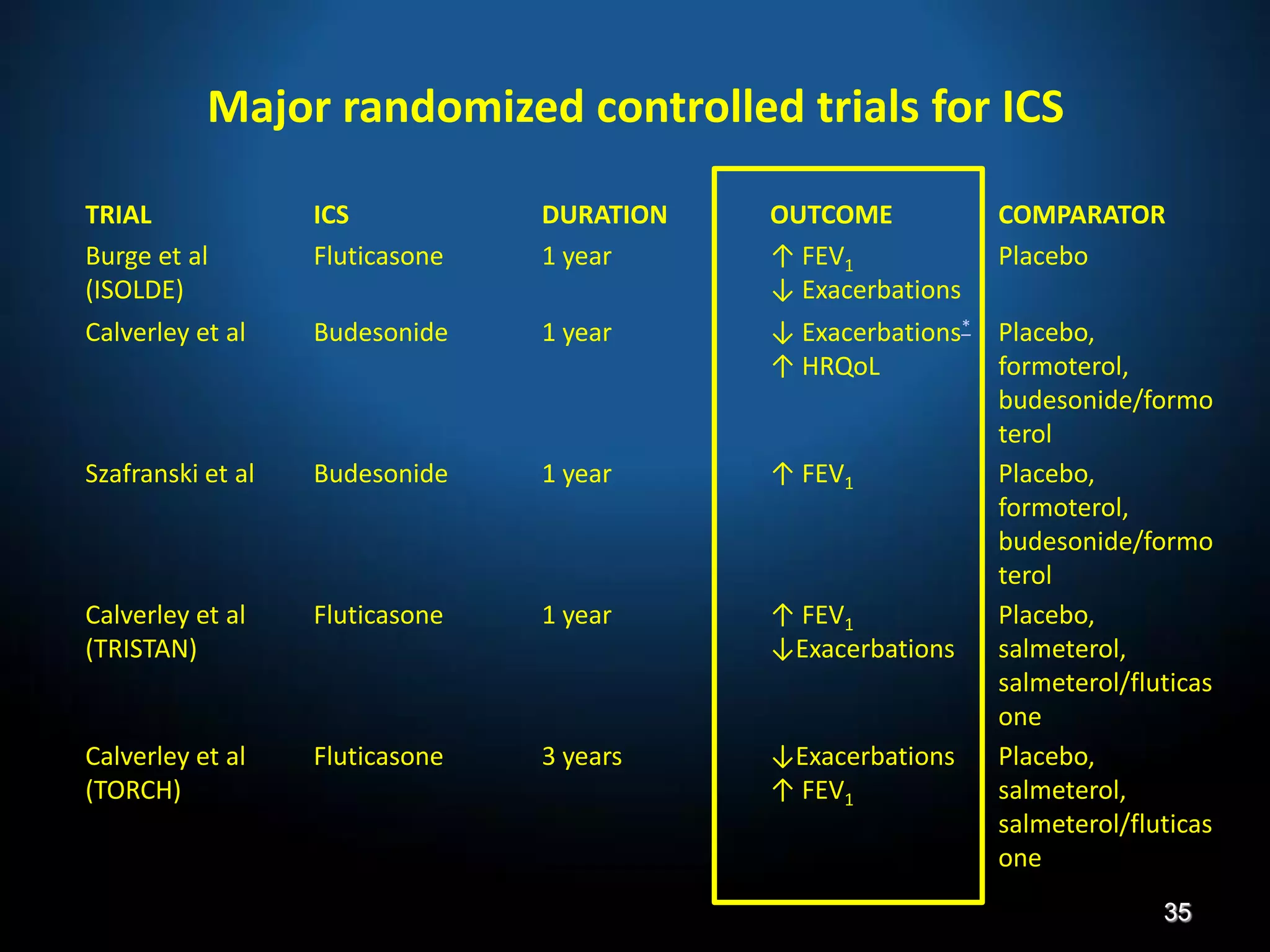
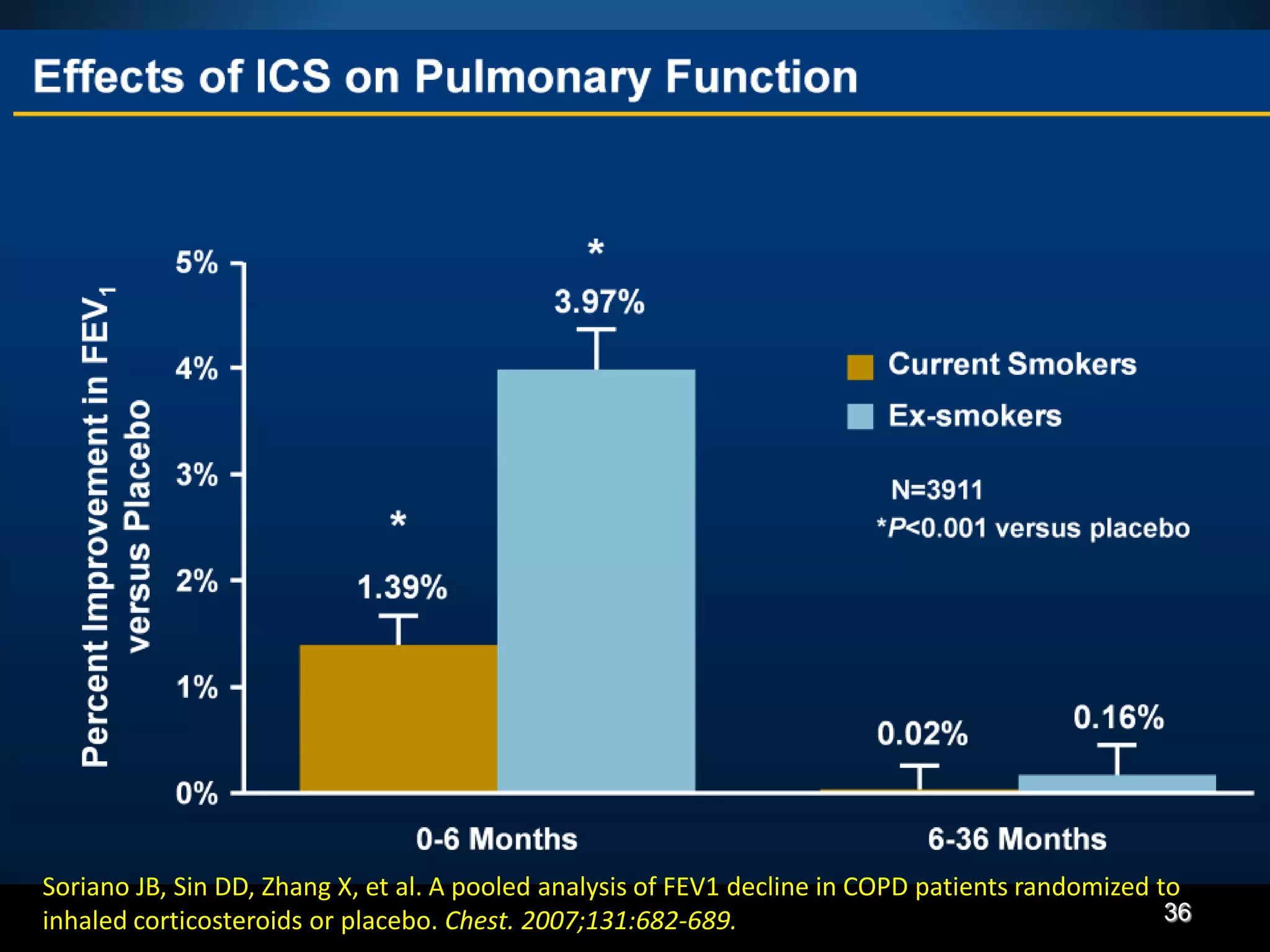
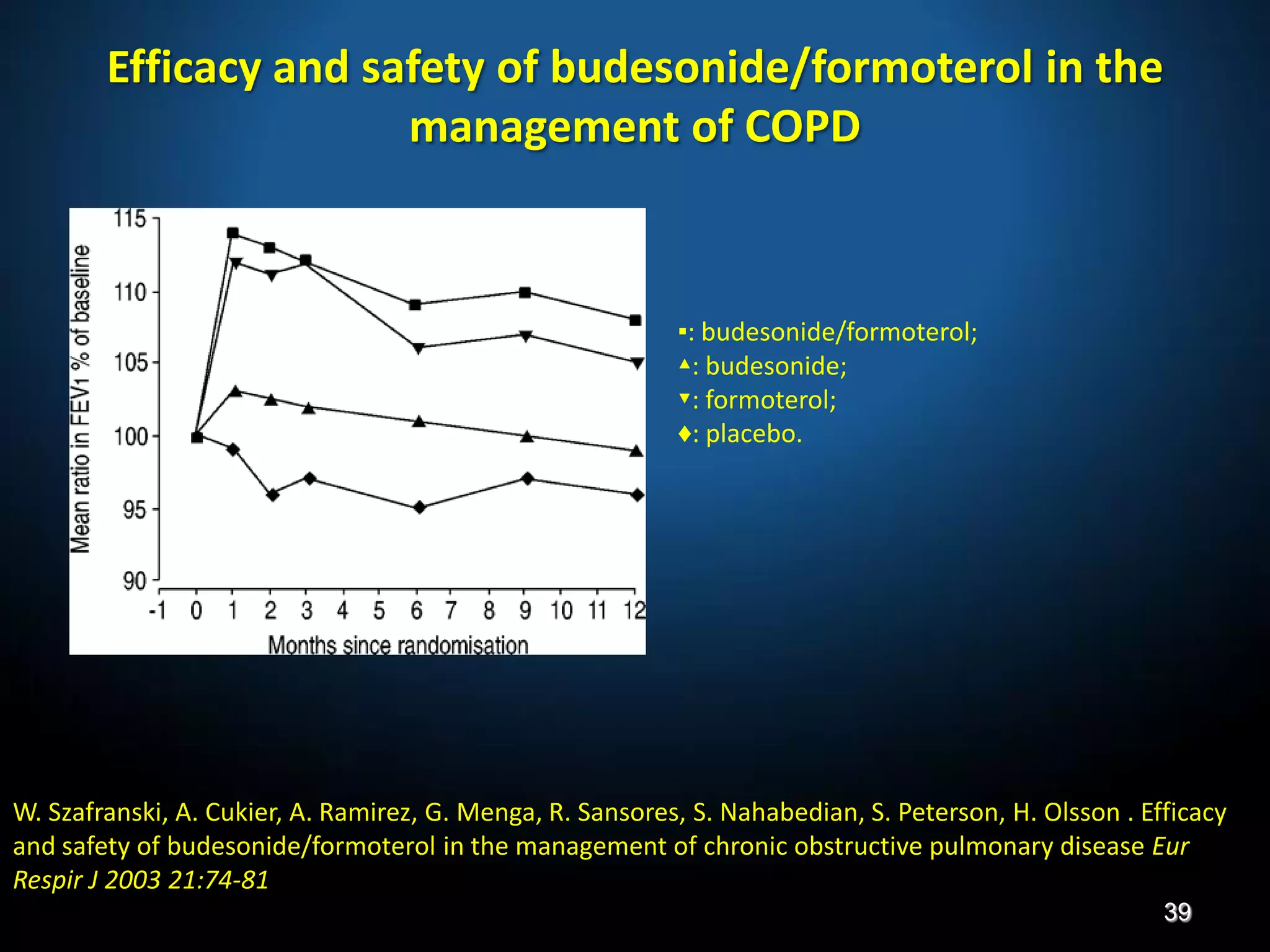
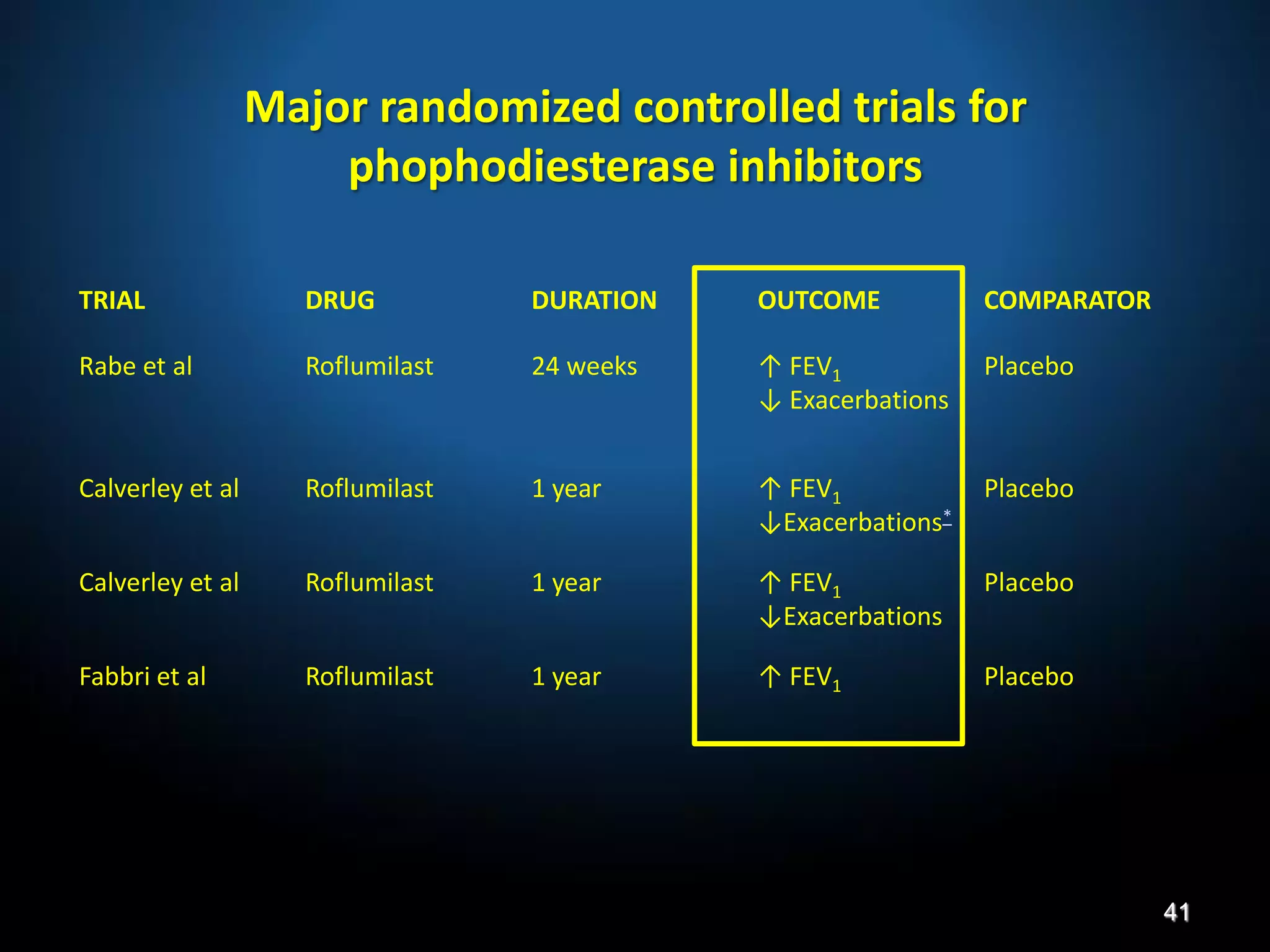
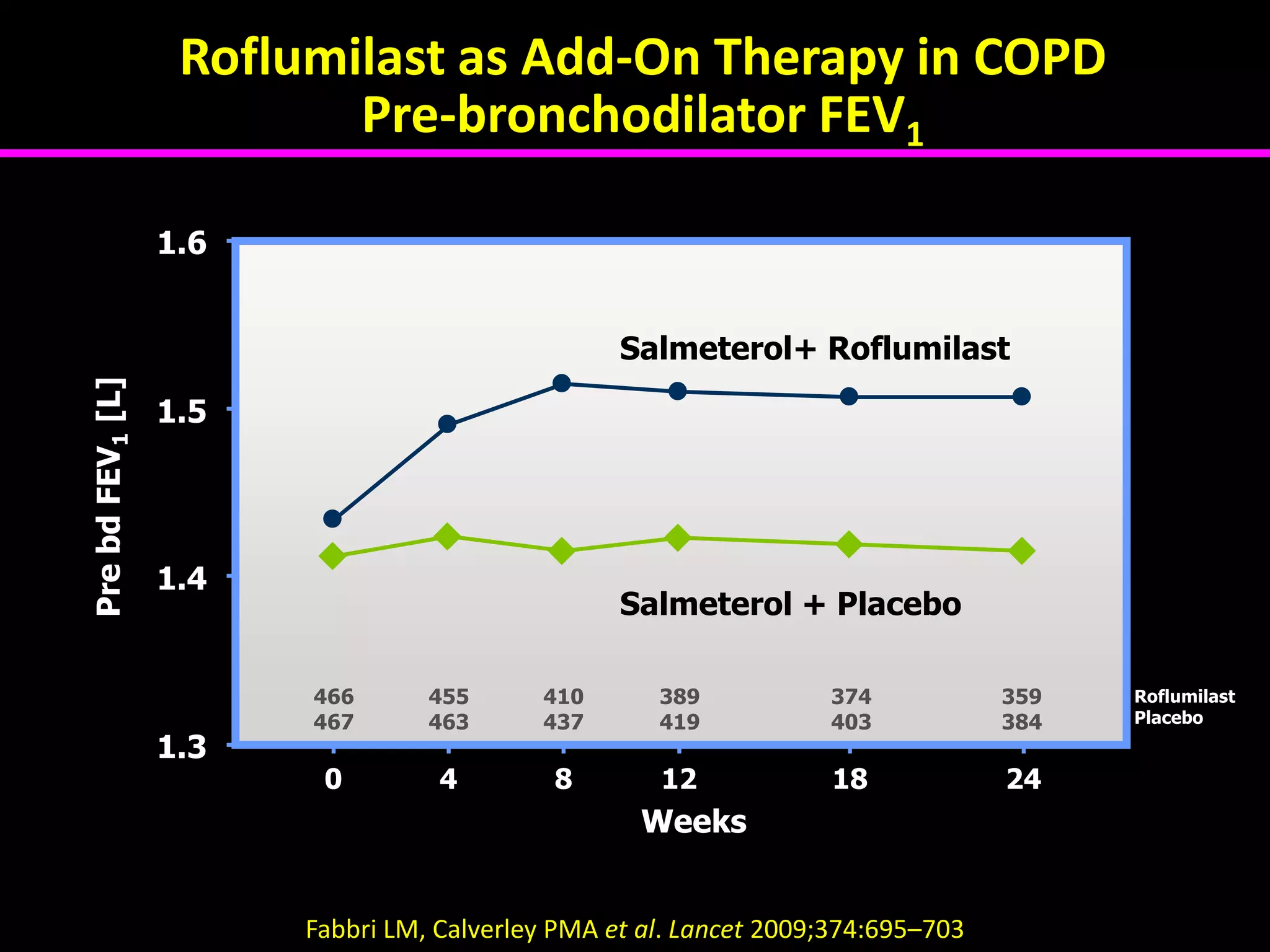
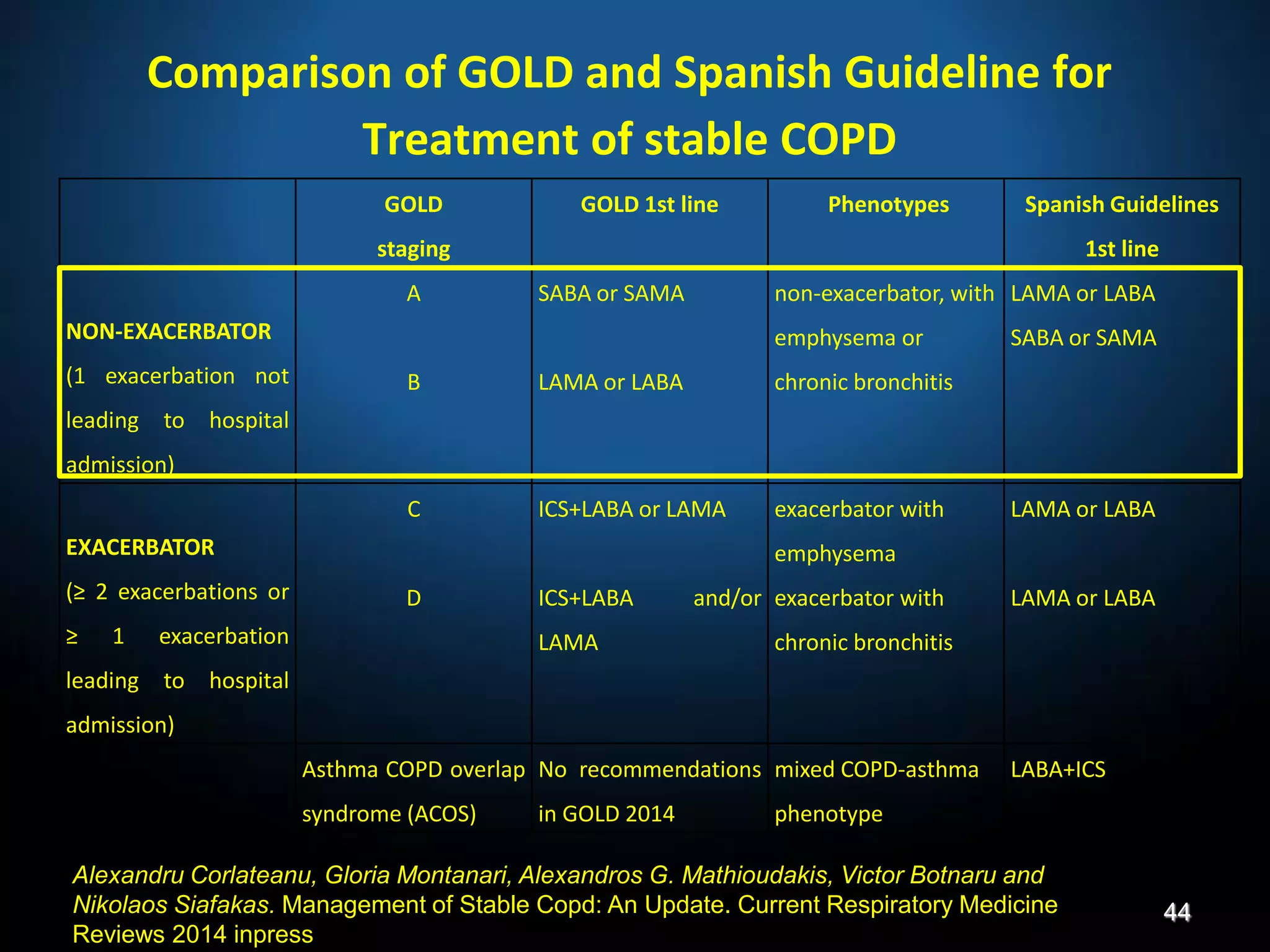
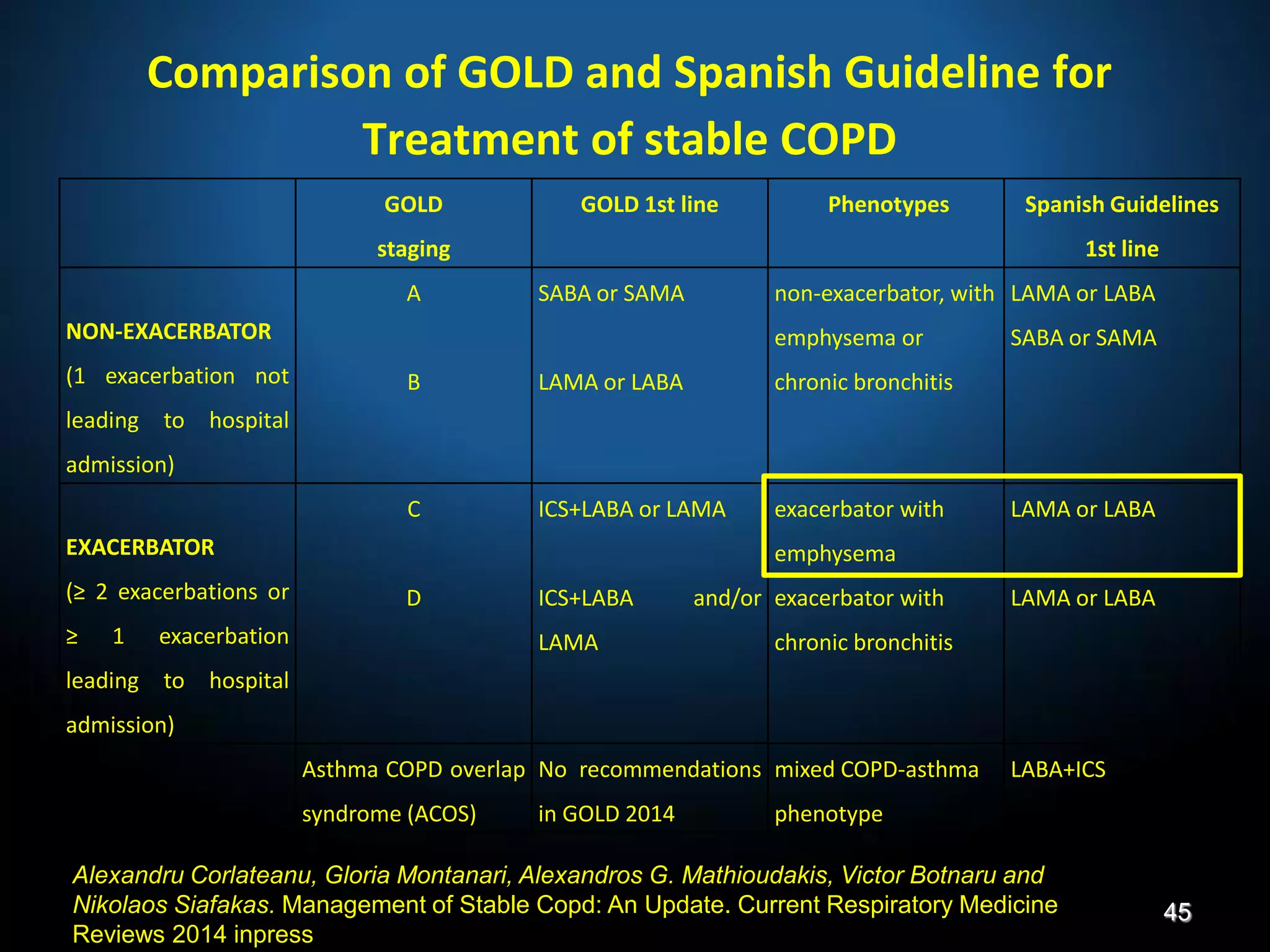
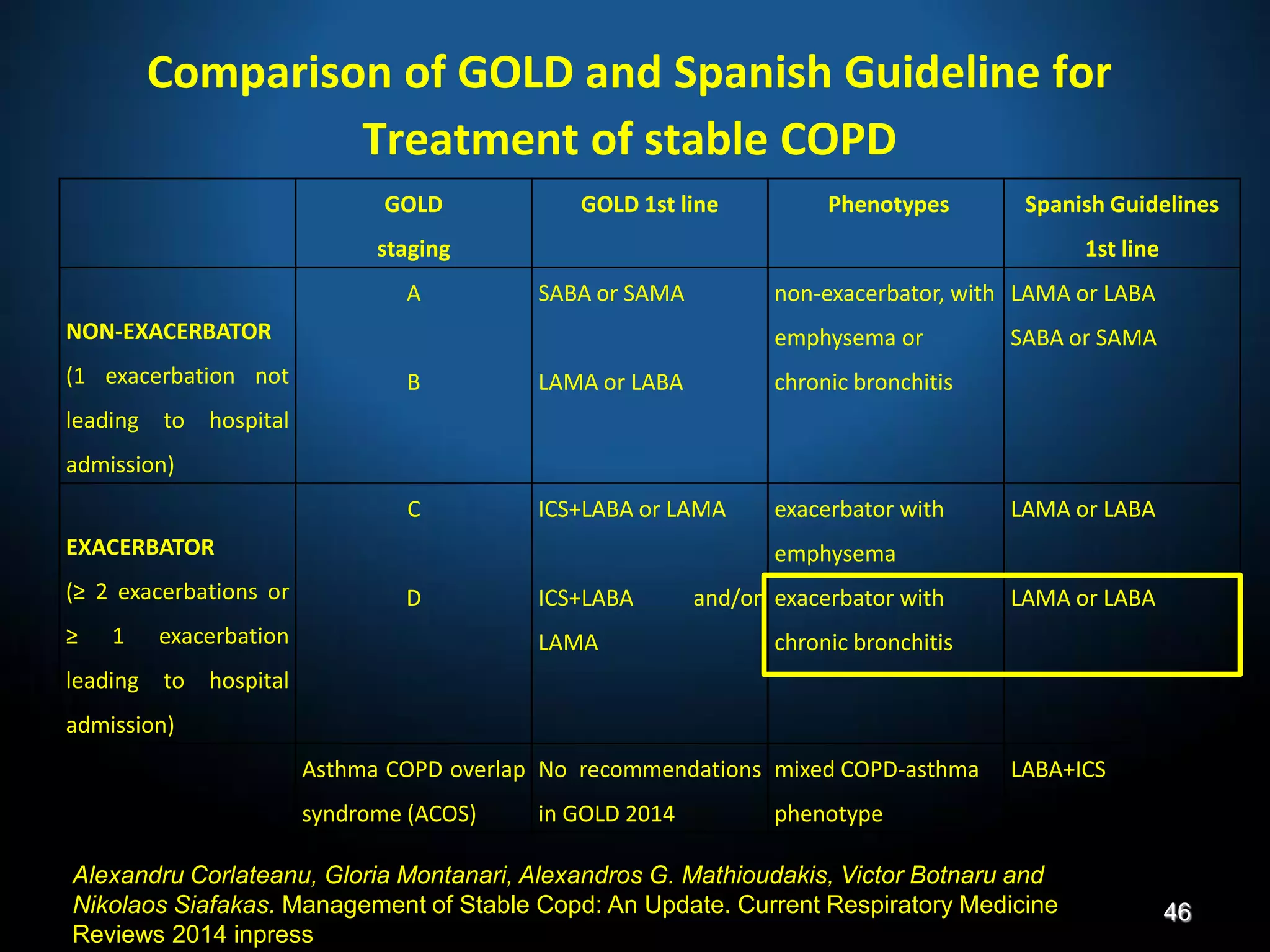
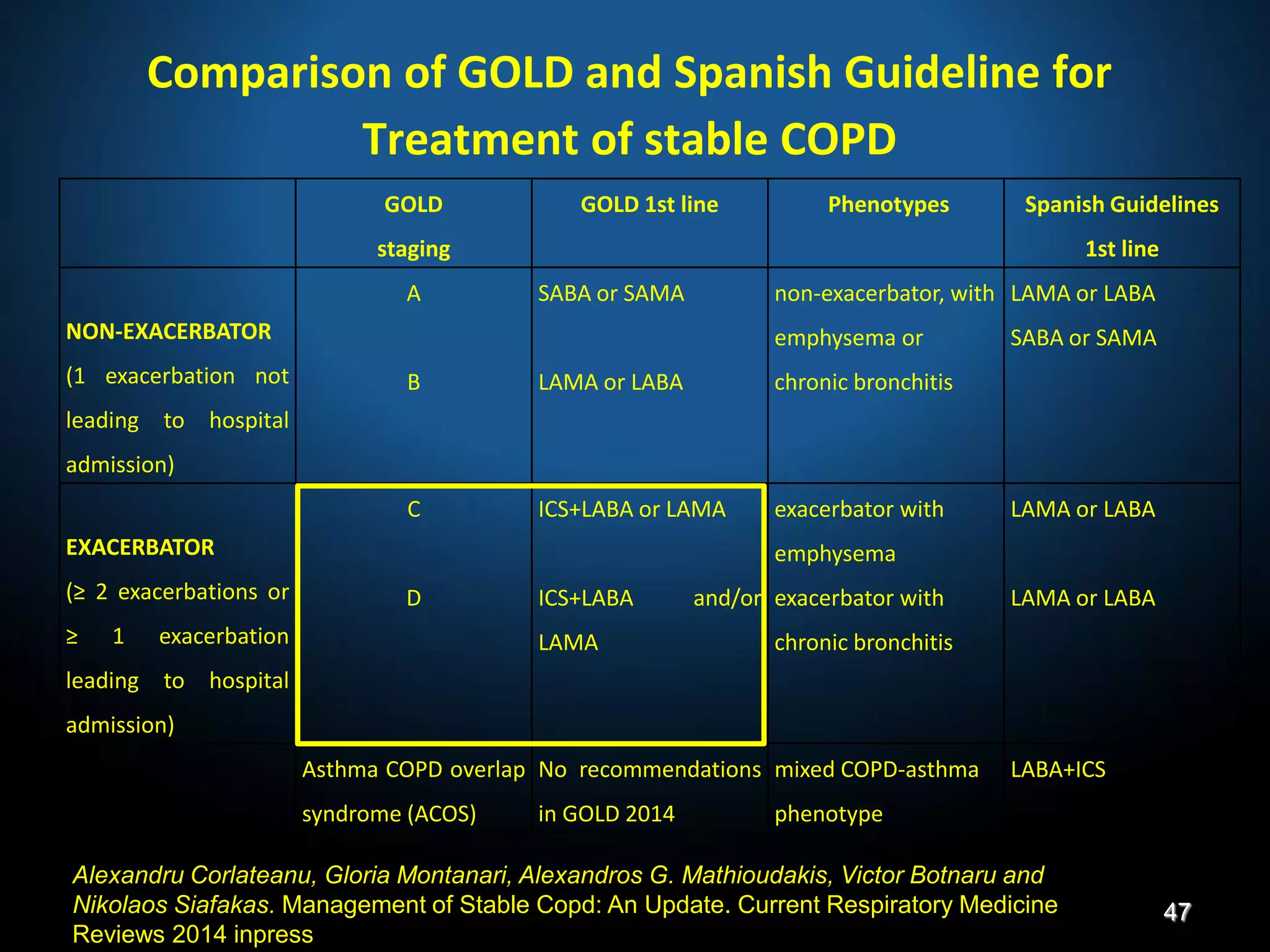
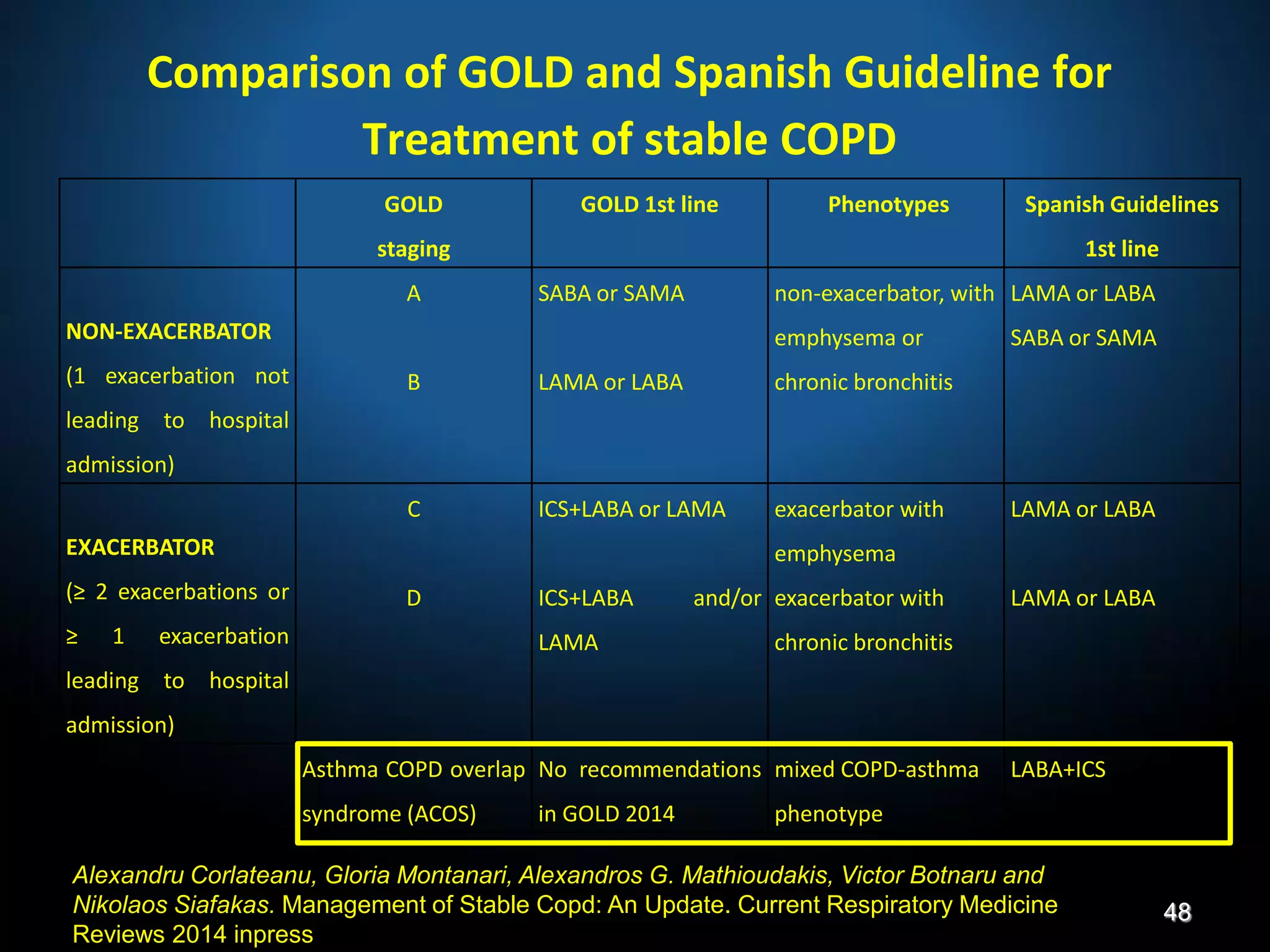
The document discusses various approaches to assessing and managing stable COPD, including assessments of severity, phenotypes, and multilateral evaluation. It reviews markers used in different assessment approaches, such as FEV1, symptoms, health status, and exacerbation risk. Non-pharmacological treatments like smoking cessation, pulmonary rehabilitation, and vaccinations are outlined. The efficacy and safety of various pharmacological treatments is summarized based on major randomized controlled trials, including long-acting bronchodilators, inhaled corticosteroids, their combinations, and phosphodiesterase inhibitors. Therapeutic strategies for stable COPD are compared between GOLD guidelines and Spanish guidelines.