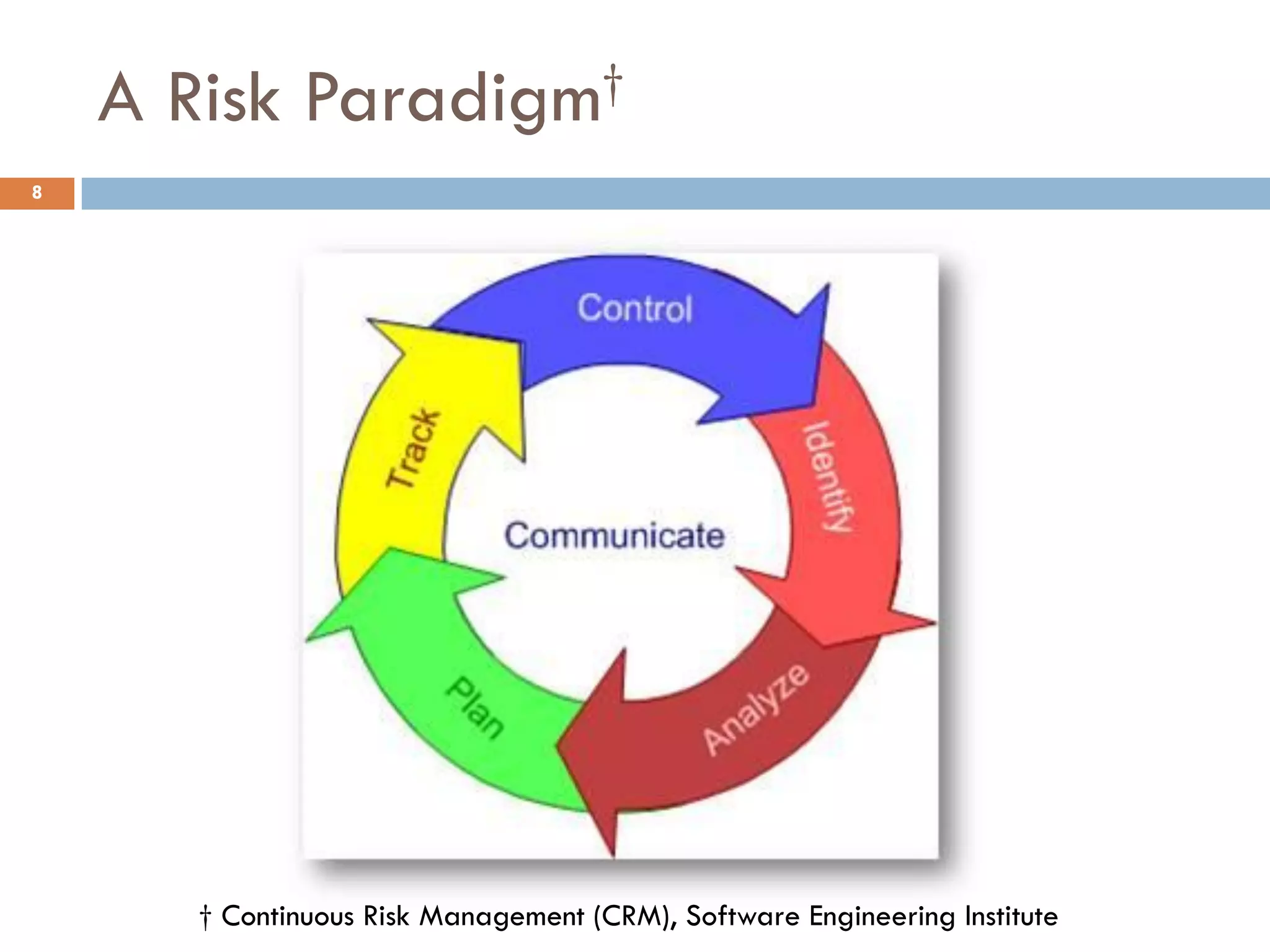
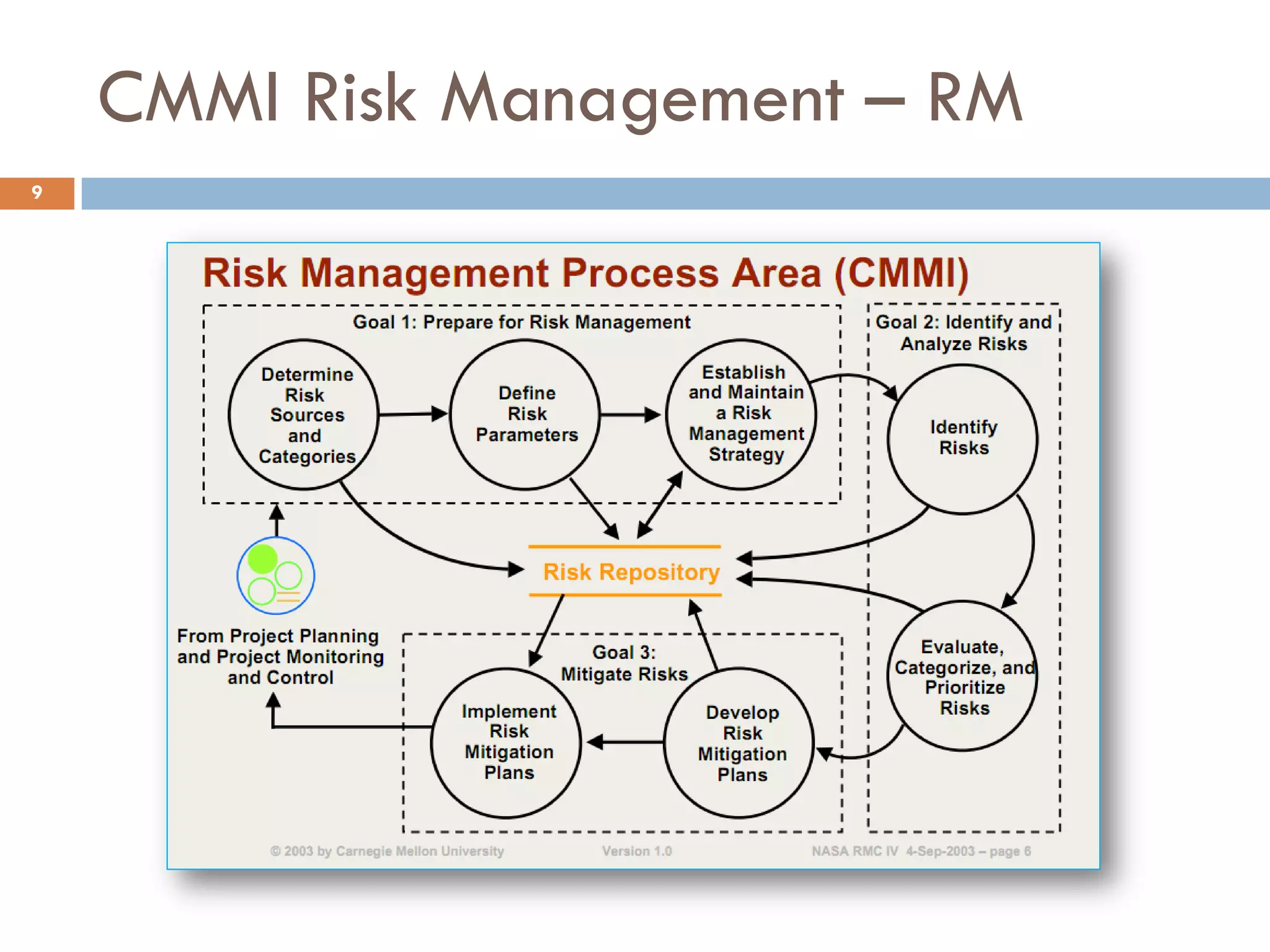
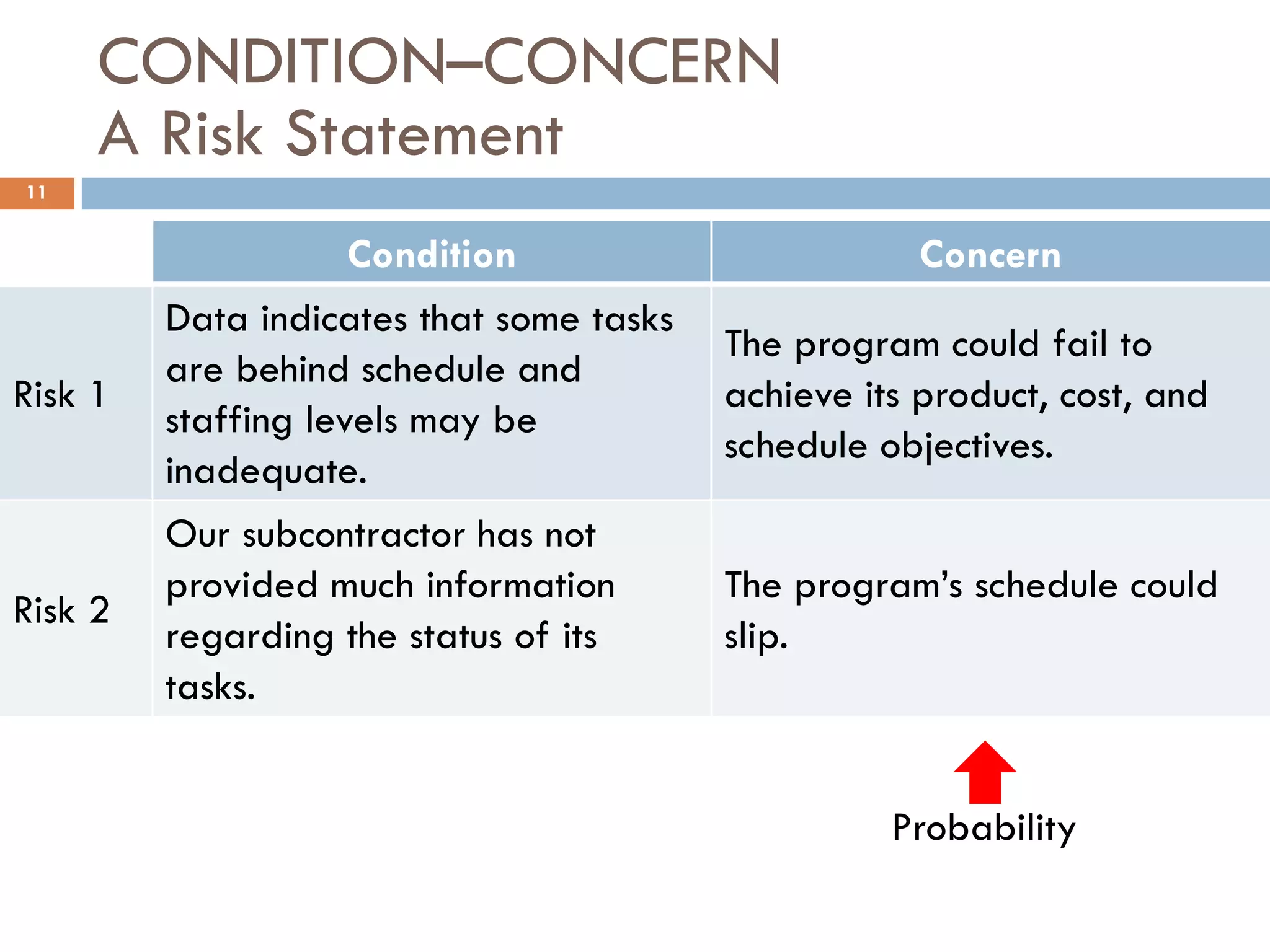
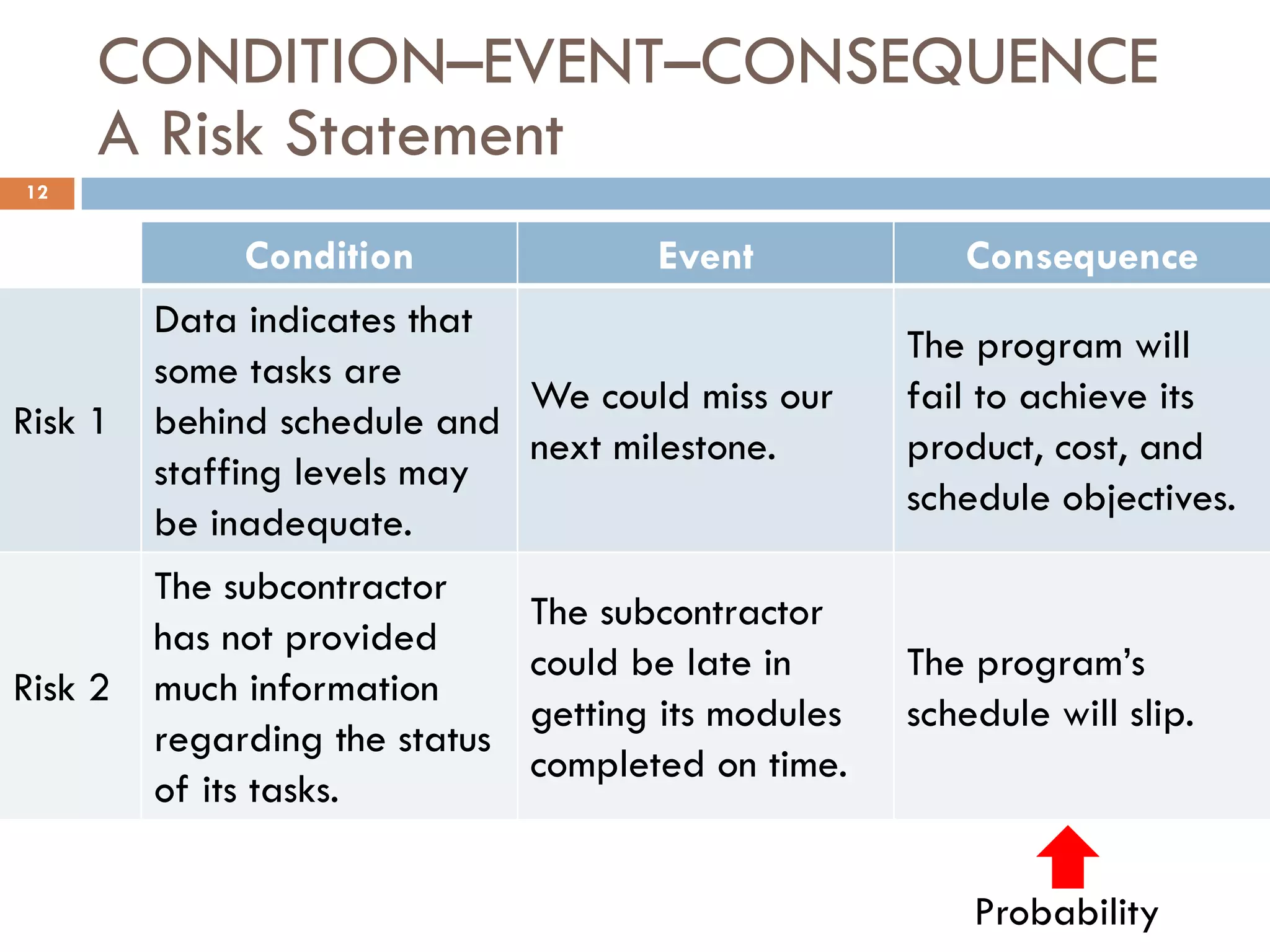
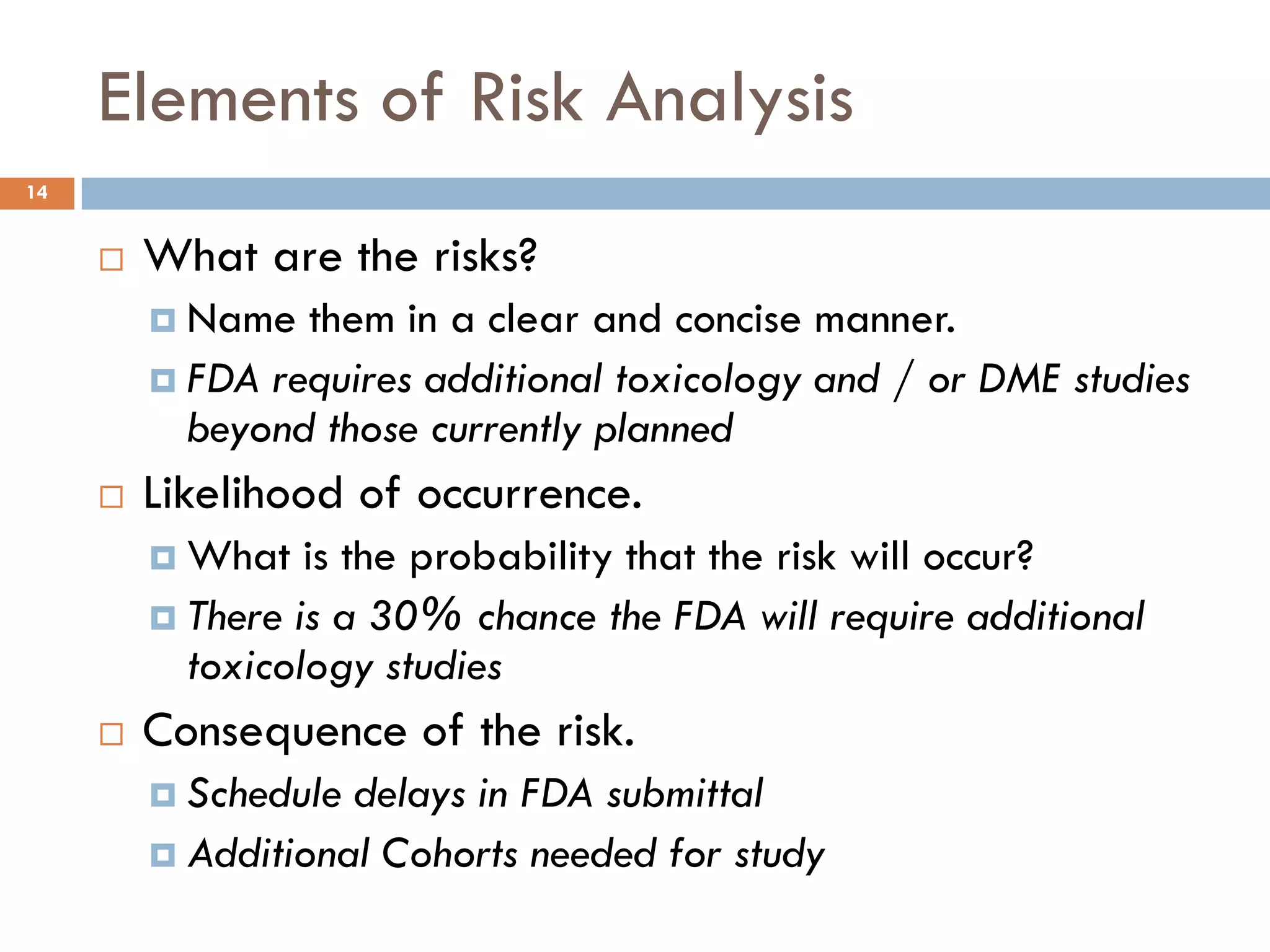
The document discusses applying risk management techniques to high-risk technology projects, defining key risk management terms like risk, uncertainty, likelihood, and consequences. It presents different frameworks for analyzing and assessing risks, including using condition-consequence or if-then statements to describe risks, and analyzing the likelihood and impact of risks in a summary grid. The goal is to help project managers make sense of the many types of risks that can affect projects and prioritize them for mitigation.






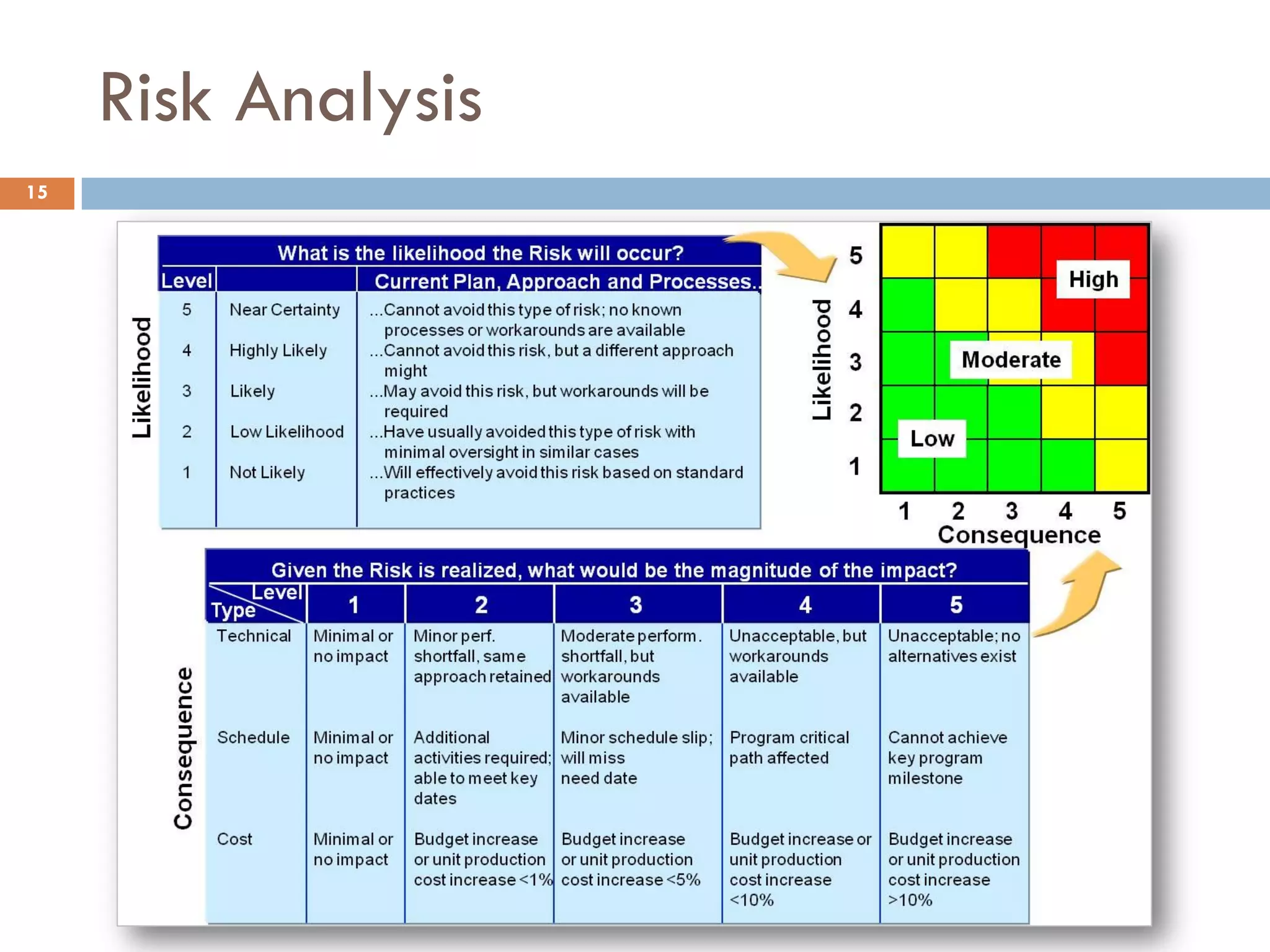
![Example Risk Summary Grid
16
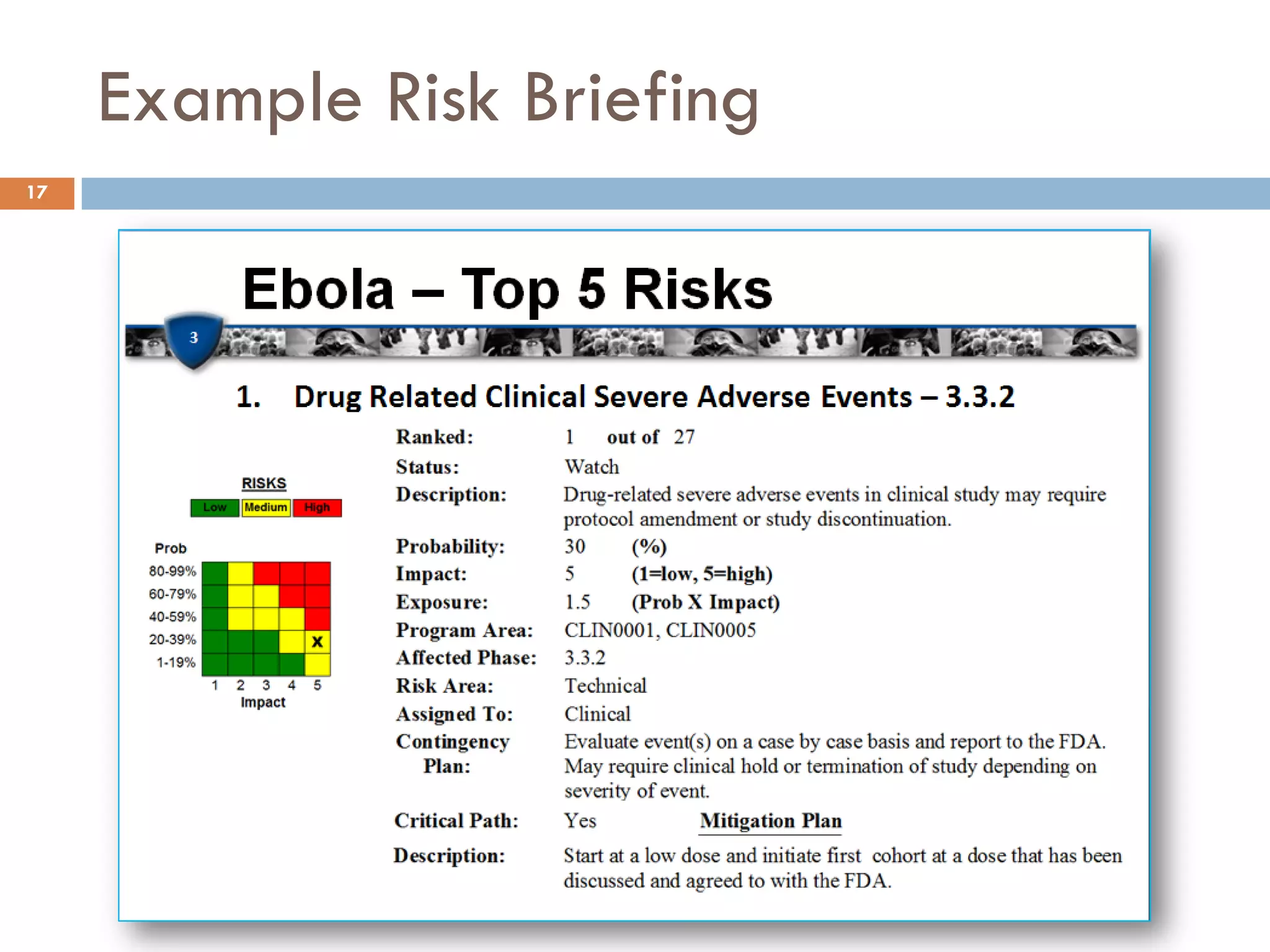
1. FDA requires additional toxicology
and/or ADME studies 15. Unsuccessful synthesis 16. GLP compliance at BSL–4
2. FDA requires PK in pivotal animal scale–up from 50L to 300L USAMRIID required for The Animal
studies 16. New impurities appear as a Rule
result of scale up
3. Insufficient subunit purification at
vendor 17. Two Segment II tox studies in
4. Failure of purification equipment at non–rodent and/or Segment I and
J–M Segment III studies required for
5. New impurities appear as a result of Category B label
scale up from 8L to 50L 5
6. Subunits or API temporarily
unavailable 18. FDA demands aerosol exposure
4
7. Lot failures of subunits, API or drug (i.e. viral challenge) experiments
Likelihood
product be performed in nonhuman
8. One or more manufacturers not 3 primate efficacy studies [L/H]
cGMP
2
10. Irreversible kidney toxicity is seen 19. One of the pivotal animal efficacy
in a subset of healthy volunteers at 1
studies fails to achieve primary
therapeutic dose levels clinical efficacy endpoint
11. Clinical trial enrolls more slowly
than expected. 1 2 3 4 5
12. Positive signal in QTc study
Consequence
13. FDA requests clinical data in 20. No Observed Adverse Effect
Special Populations pre–licensure High Level is significantly lower than
14. FDA requests larger clinical safety Moderate expected [L/H]
database than initially proposed
Low](https://image.slidesharecdn.com/applyingriskradarv2-120629165451-phpapp02/75/Applying-risk-radar-v2-16-2048.jpg)