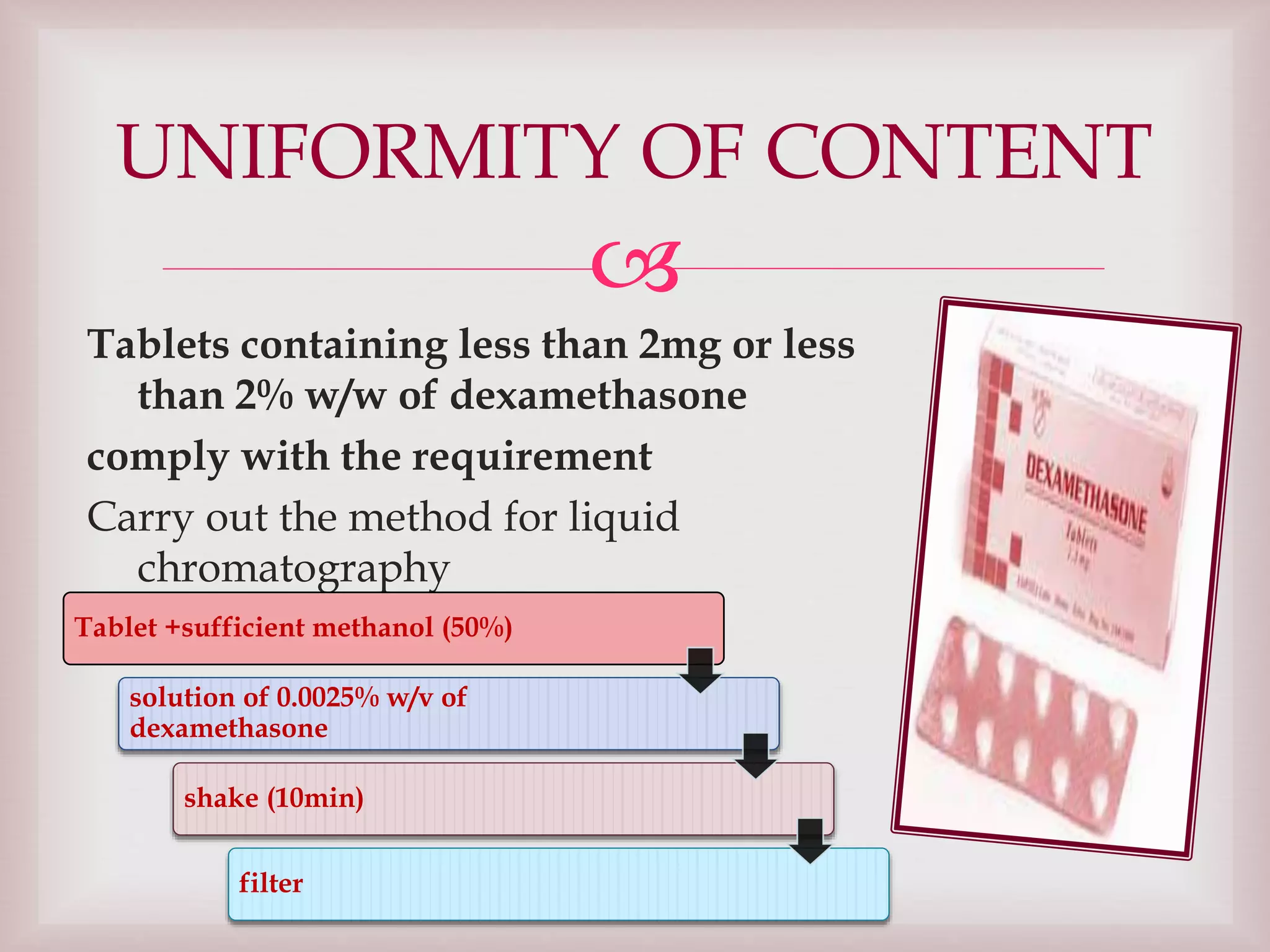
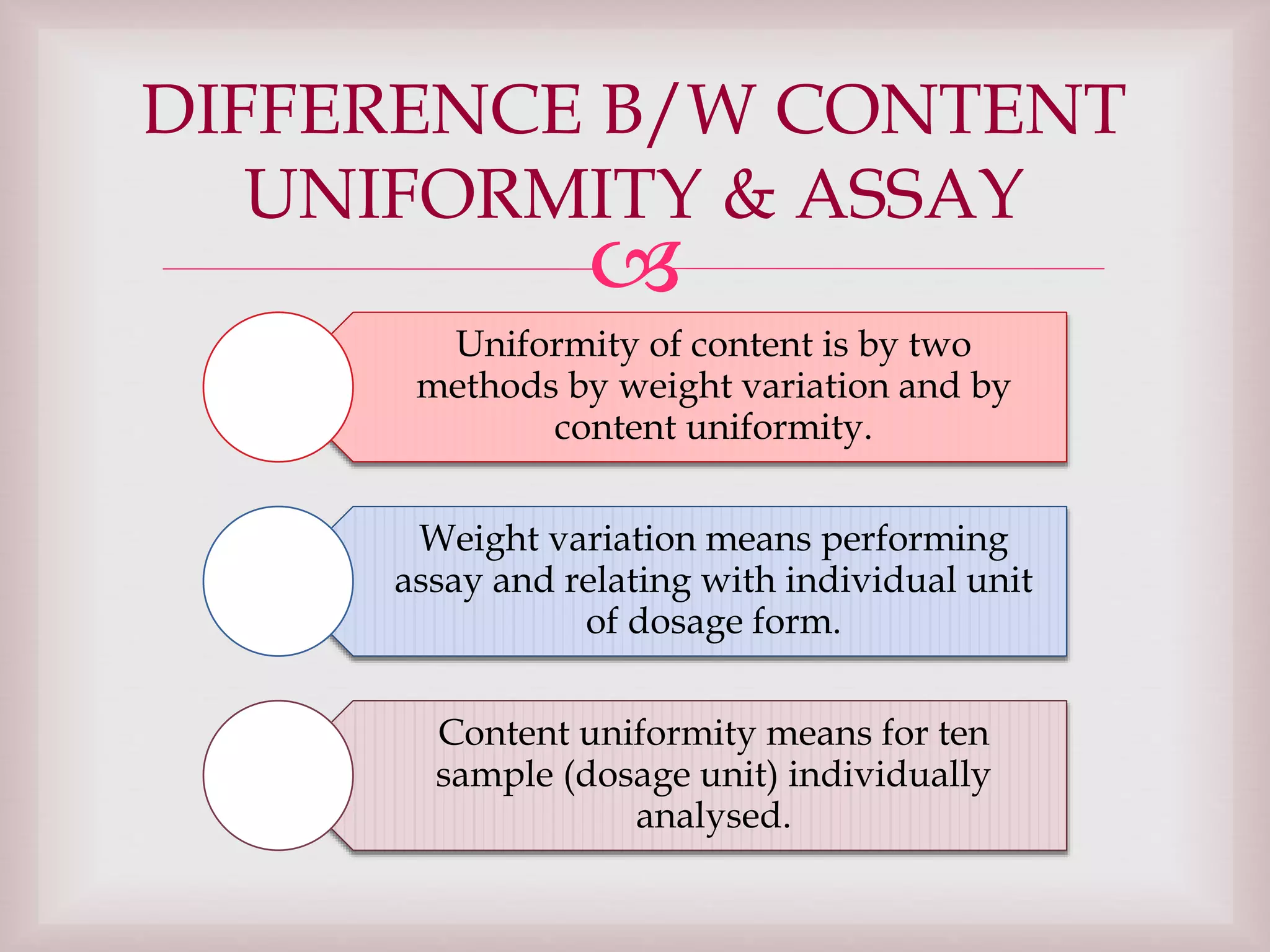
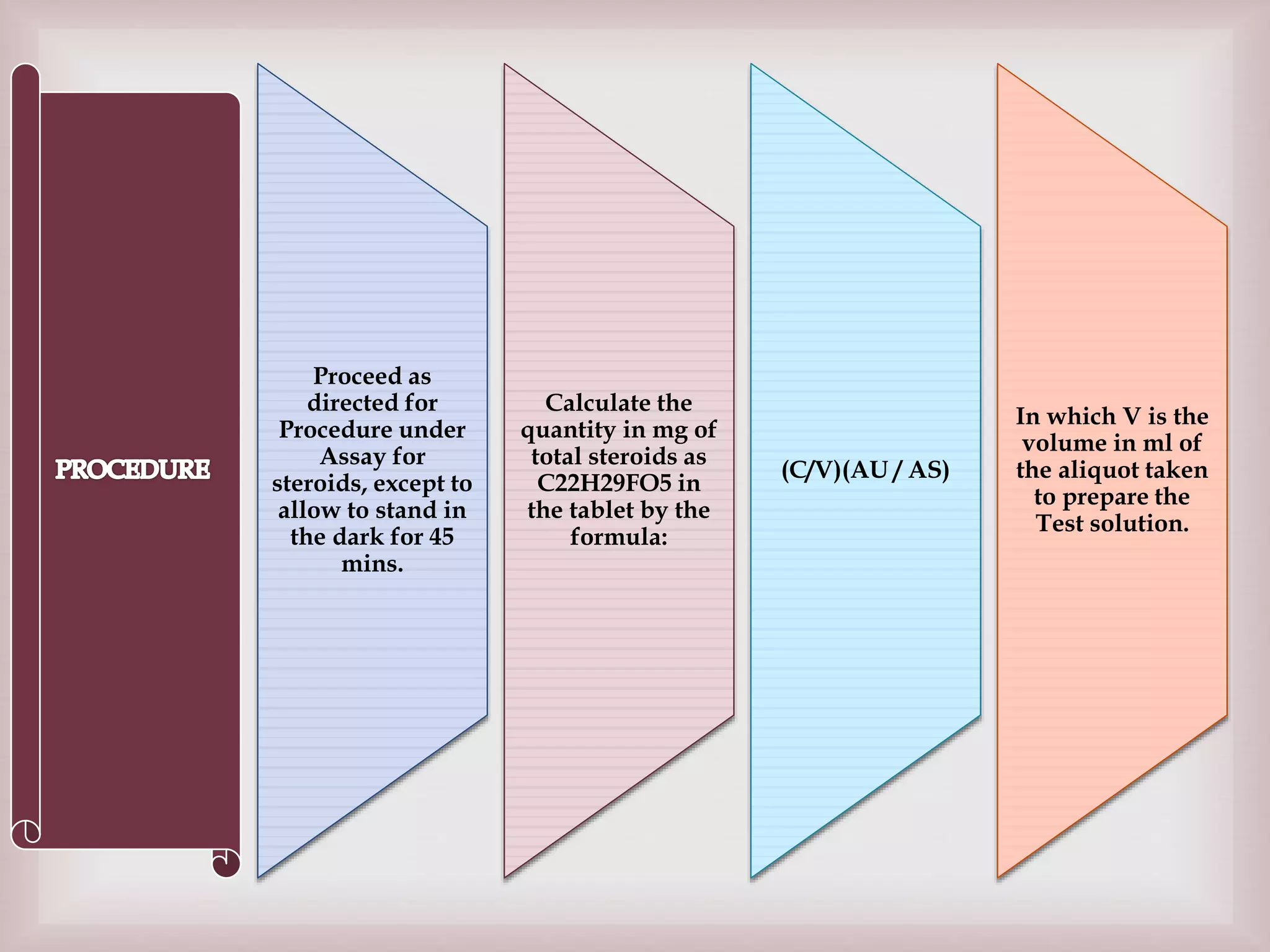
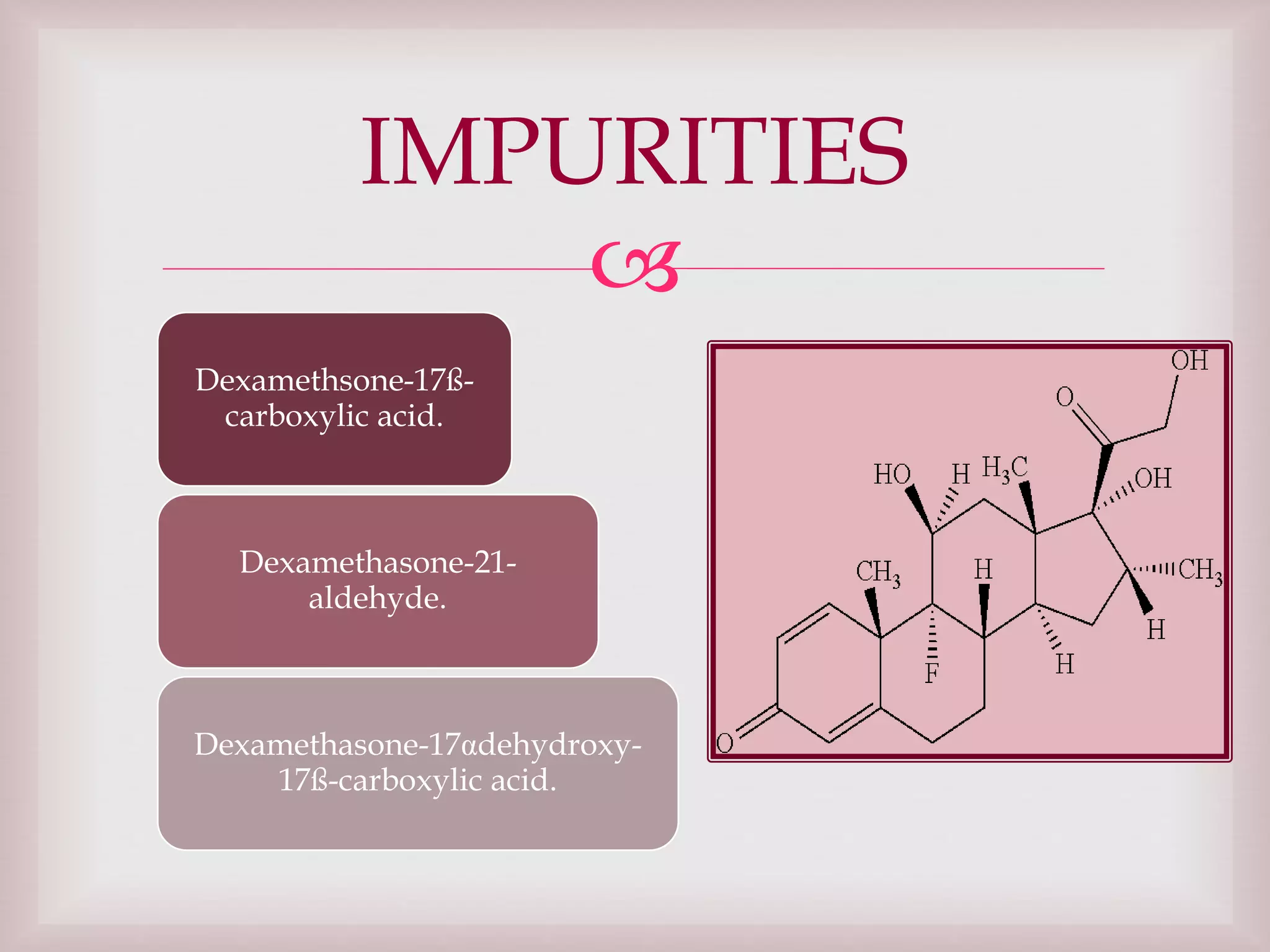
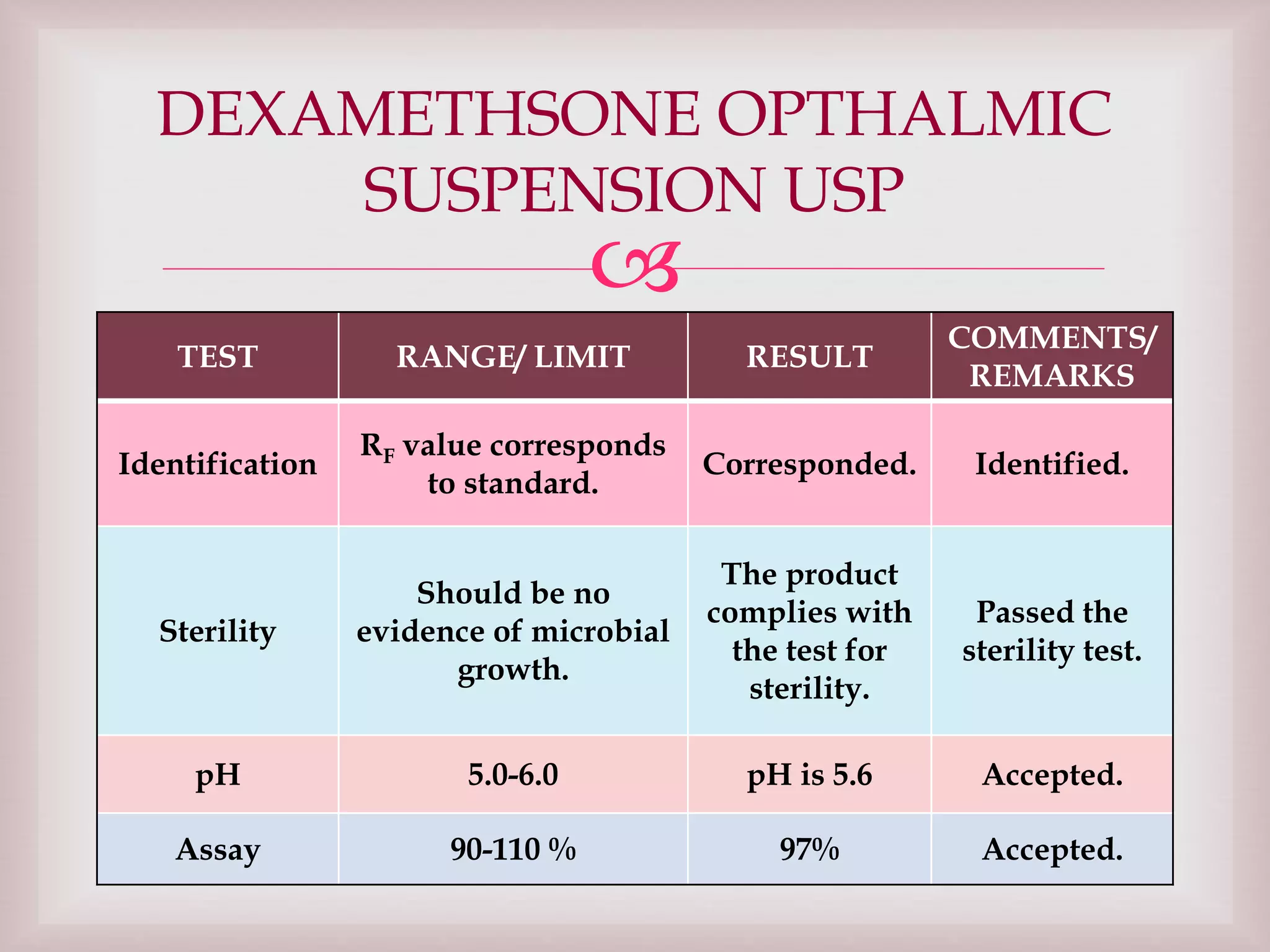
The document discusses dexamethasone, a synthetic corticosteroid, detailing its properties, manufacturing processes, testing methods, and regulatory compliance for tablet and ophthalmic suspension forms. It describes procedures for content uniformity, dissolution testing, and chromatographic assays to ensure that products contain the appropriate amount of dexamethasone and meet quality standards. It also lists specifications for packaging, storage, and testing results confirming that products comply with the required ranges for efficacy and safety.