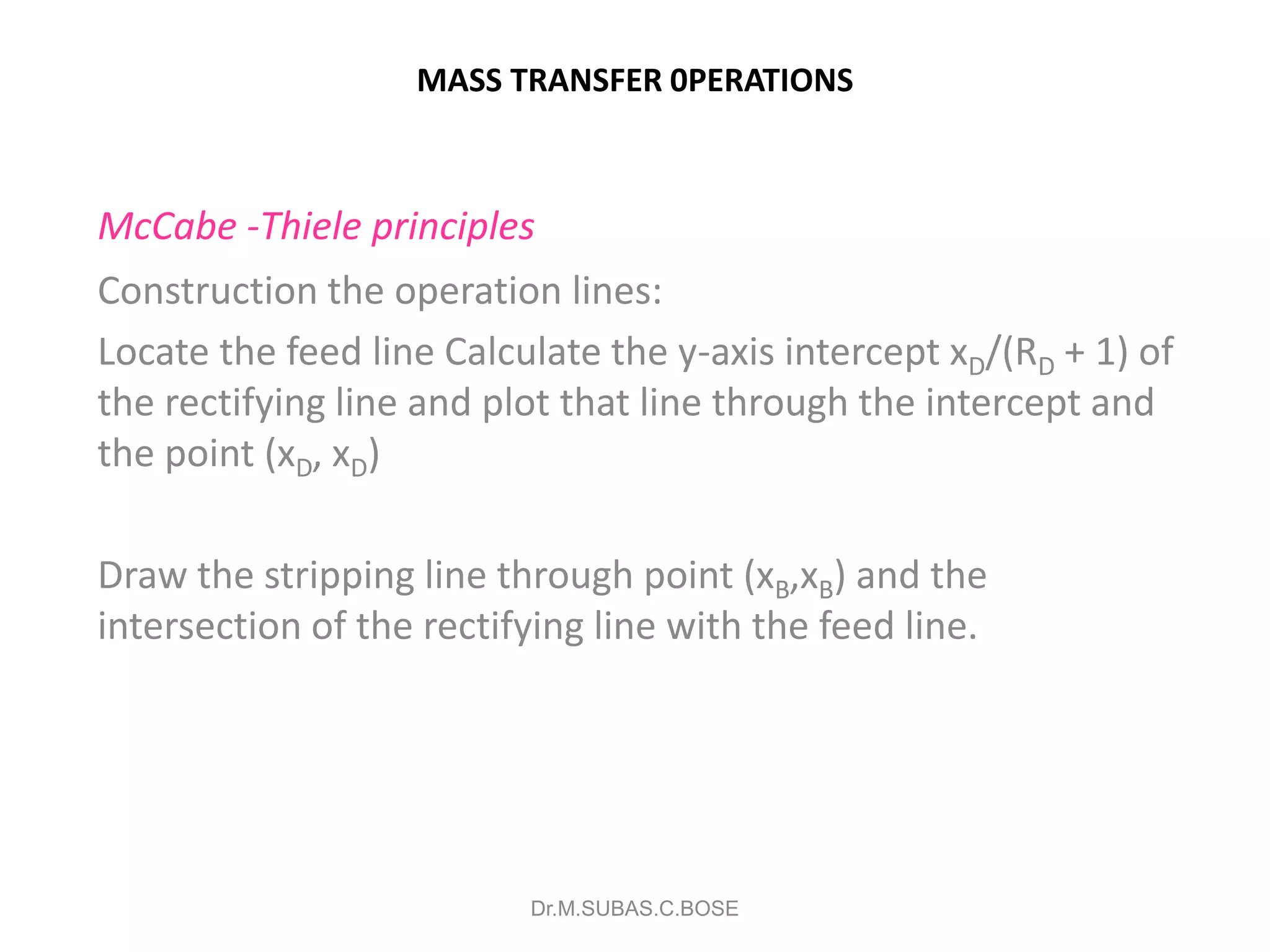
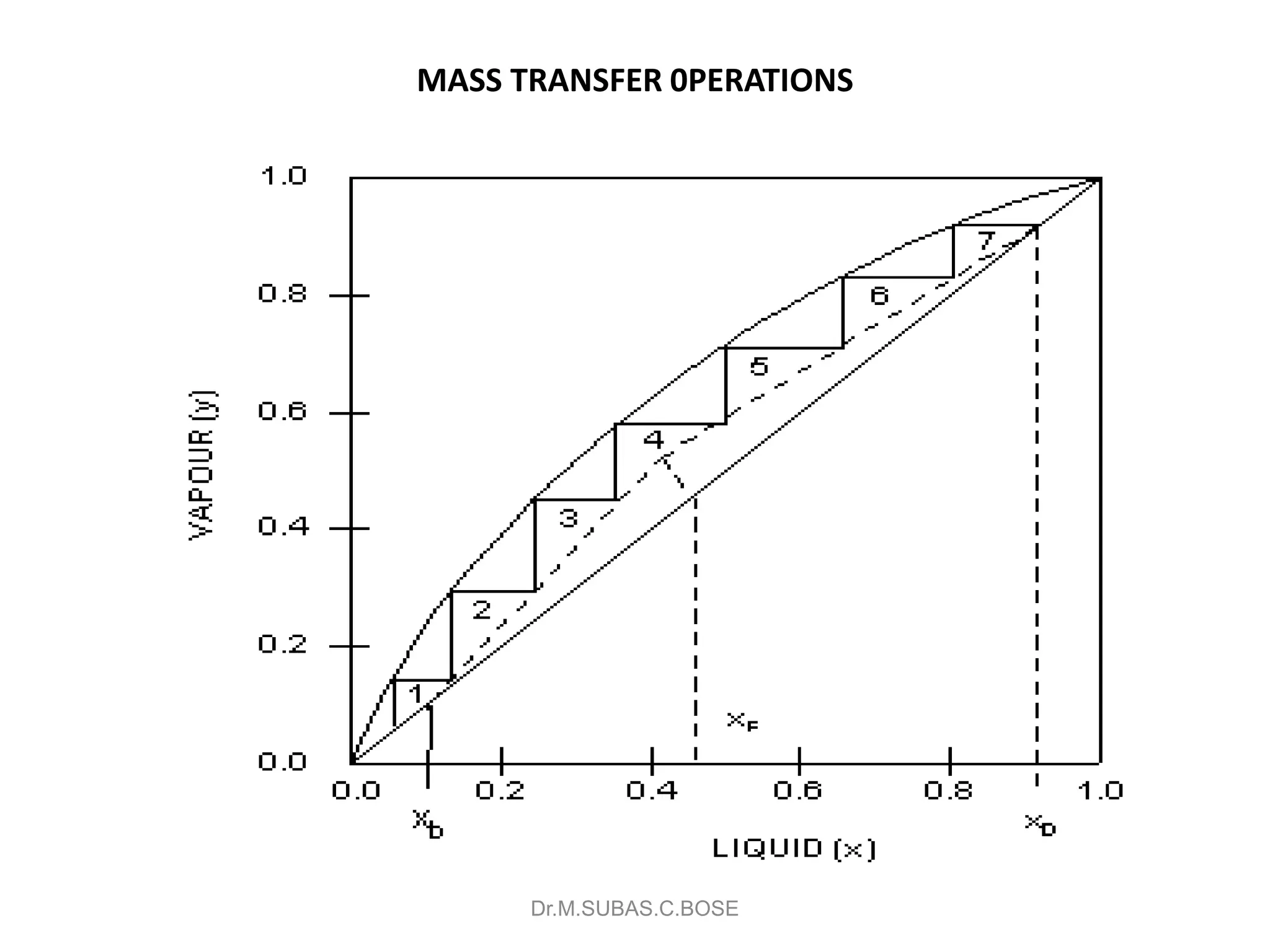
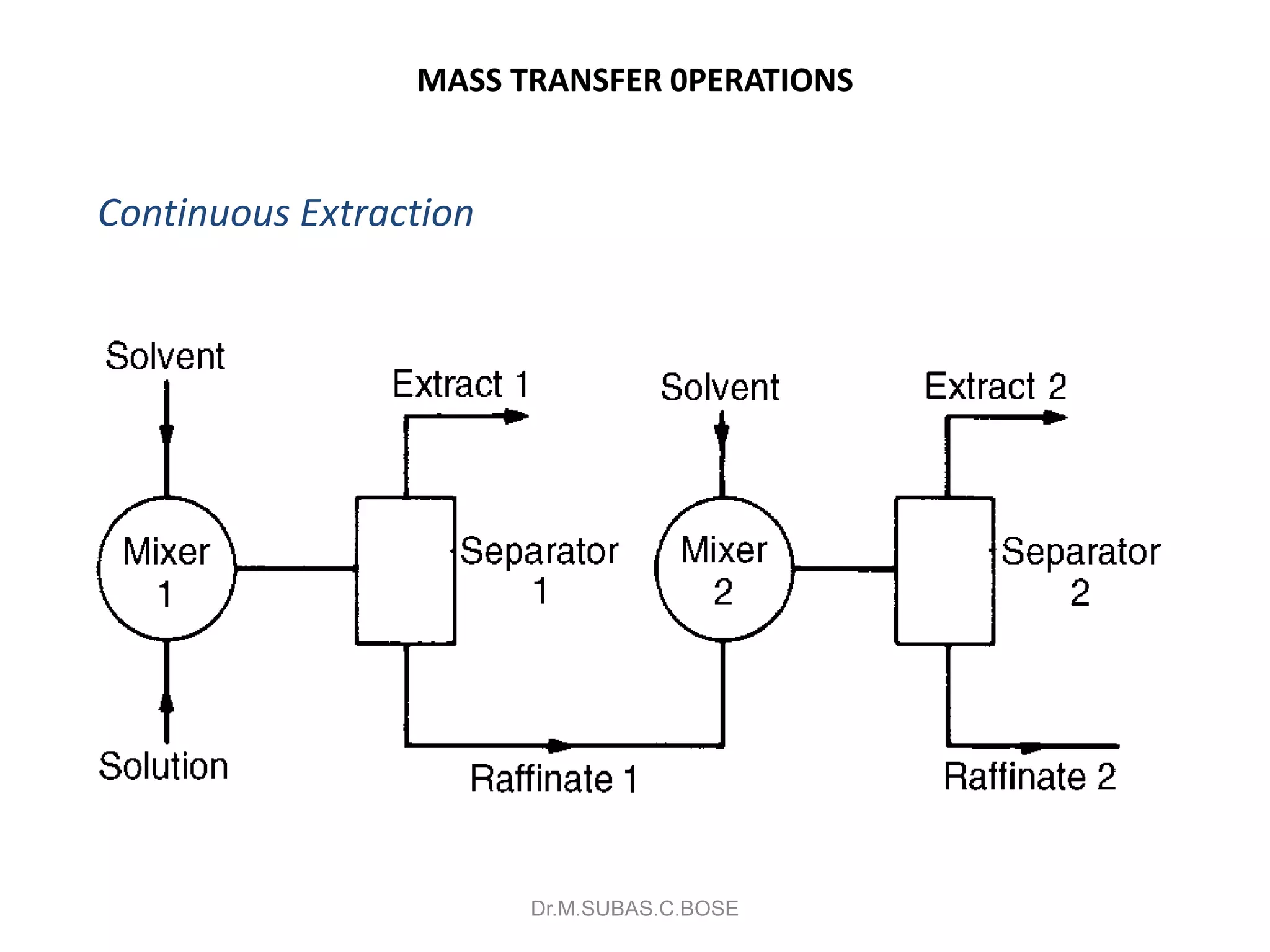
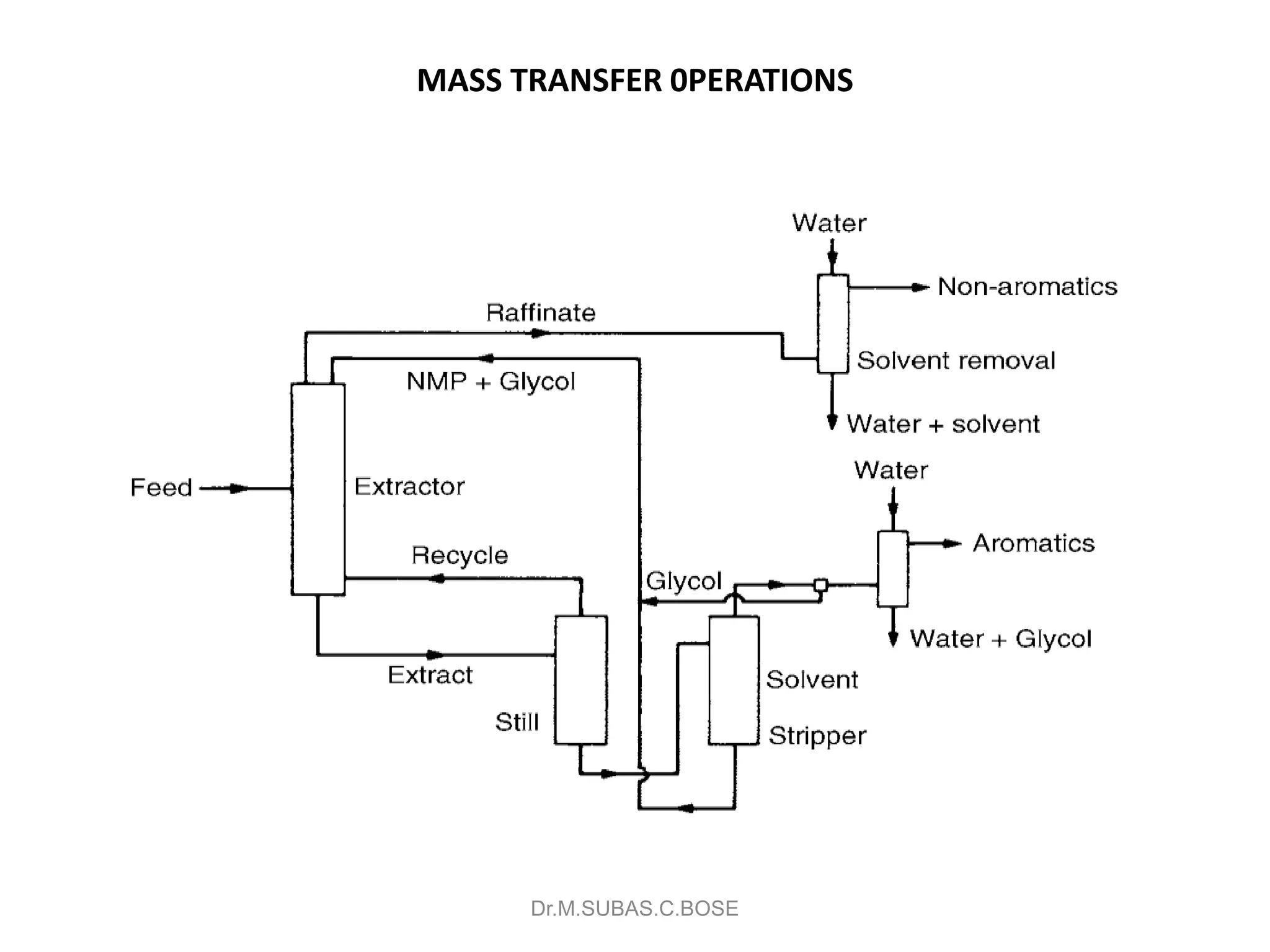
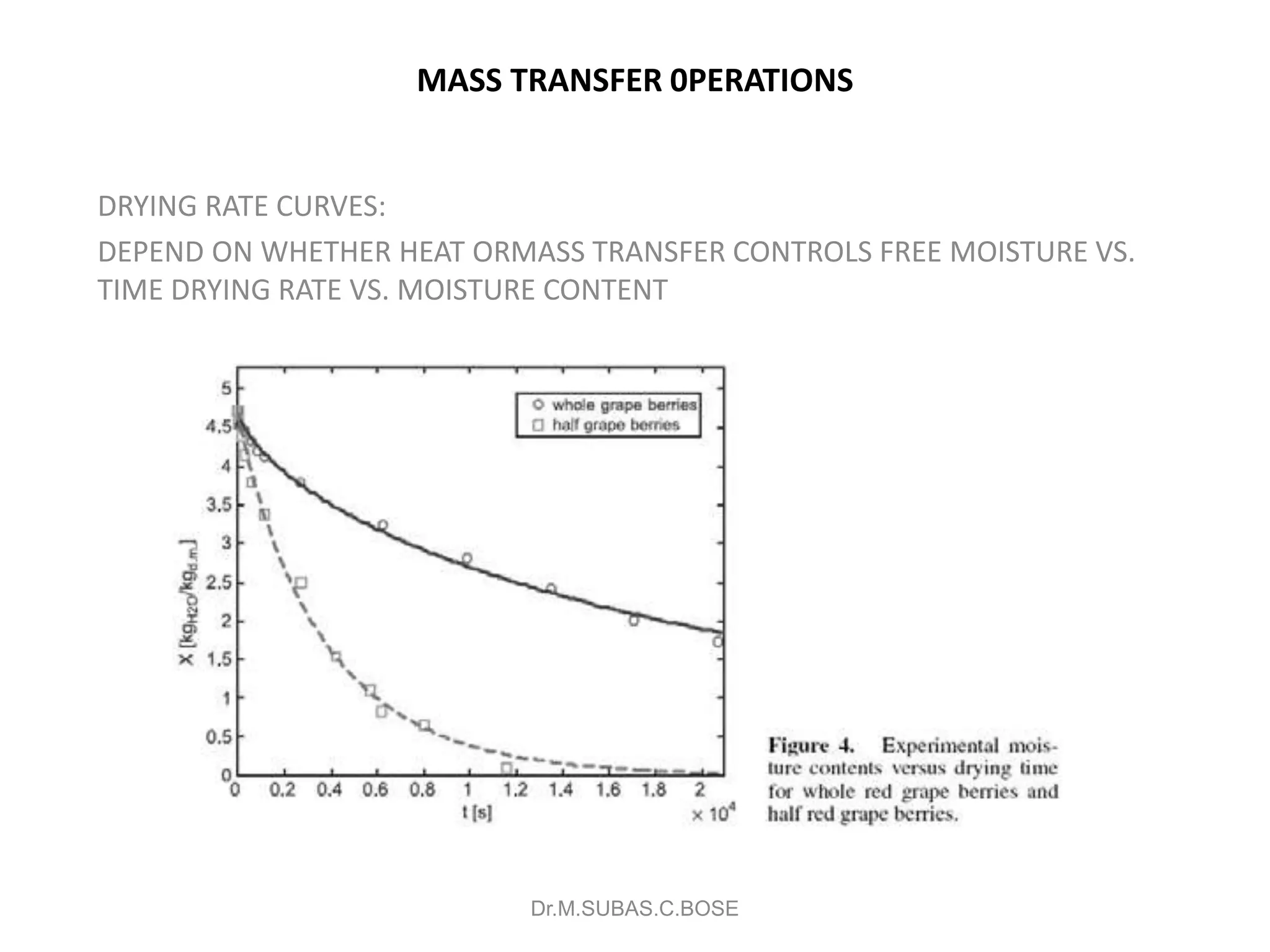
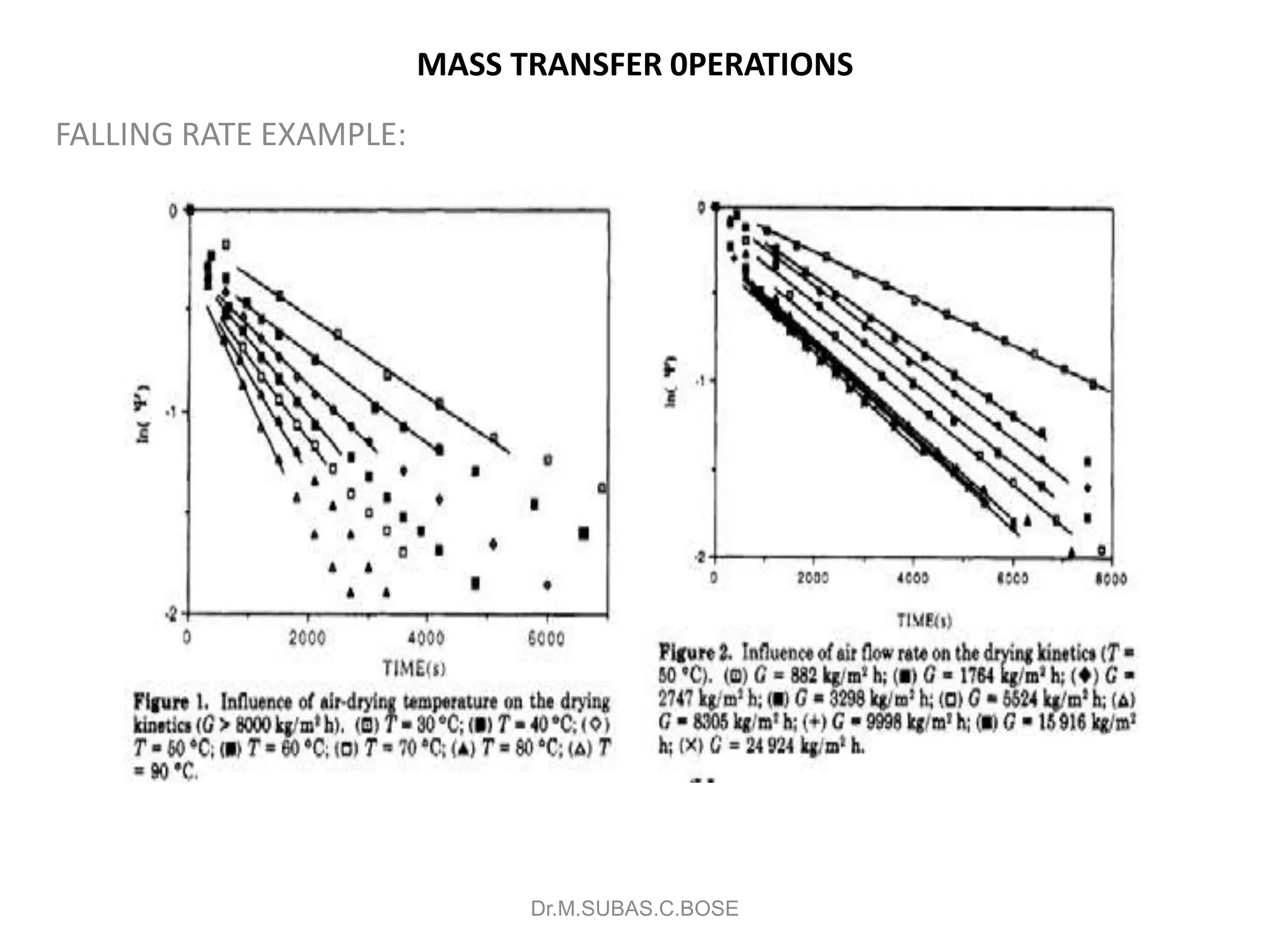
This document contains lecture notes on mass transfer operations. It discusses various topics including diffusion, gas-liquid operations like absorption, and vapor-liquid operations like distillation.
The key points covered are:
1) Diffusion is the process of mass transfer between regions of different concentrations due to random molecular motion. Pick's law describes the rate of diffusion.
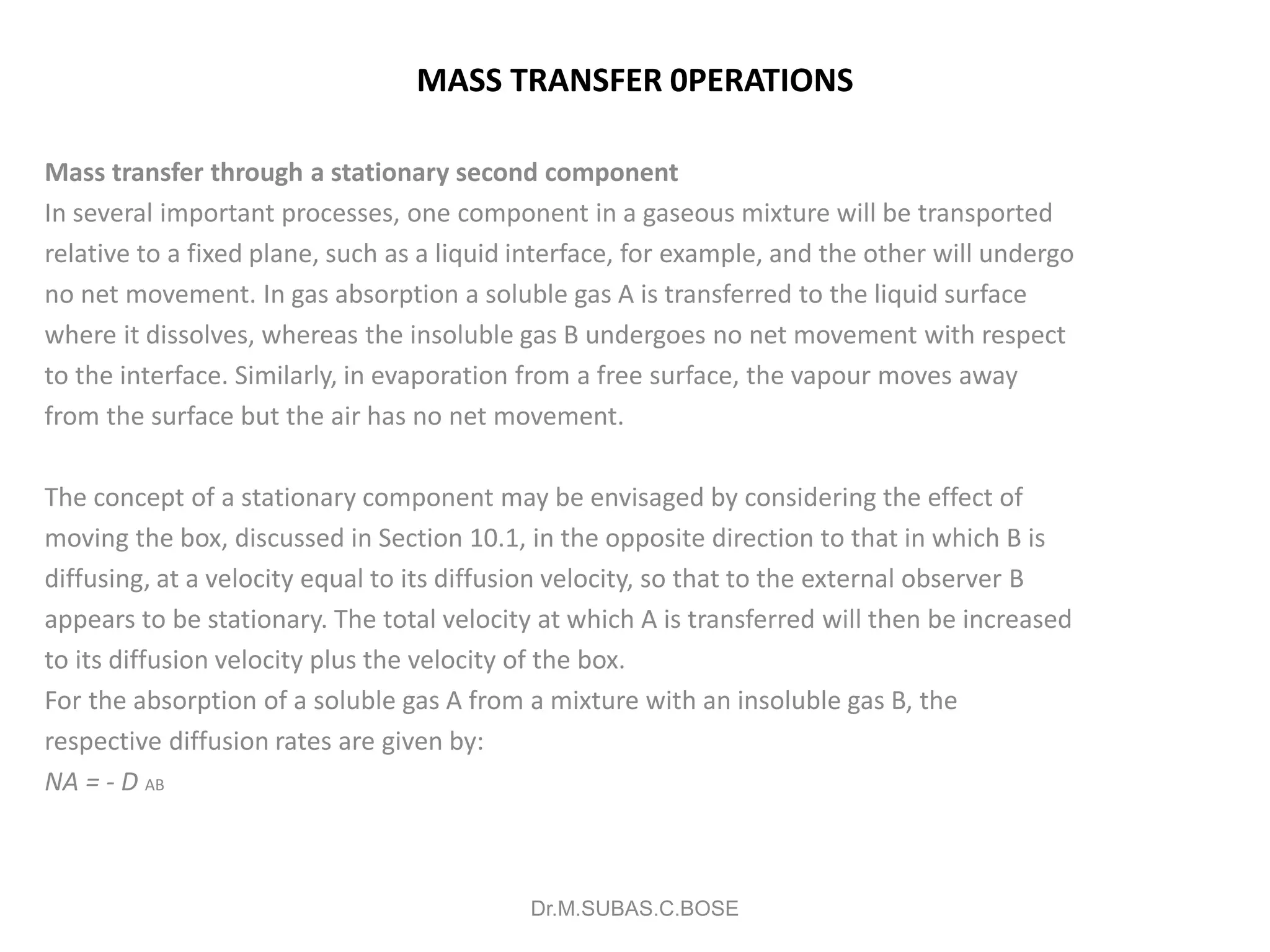
2) Gas absorption involves the removal of a soluble gas from a mixture by absorption into a liquid. Absorption towers can be analyzed using the concept of gas transfer units.
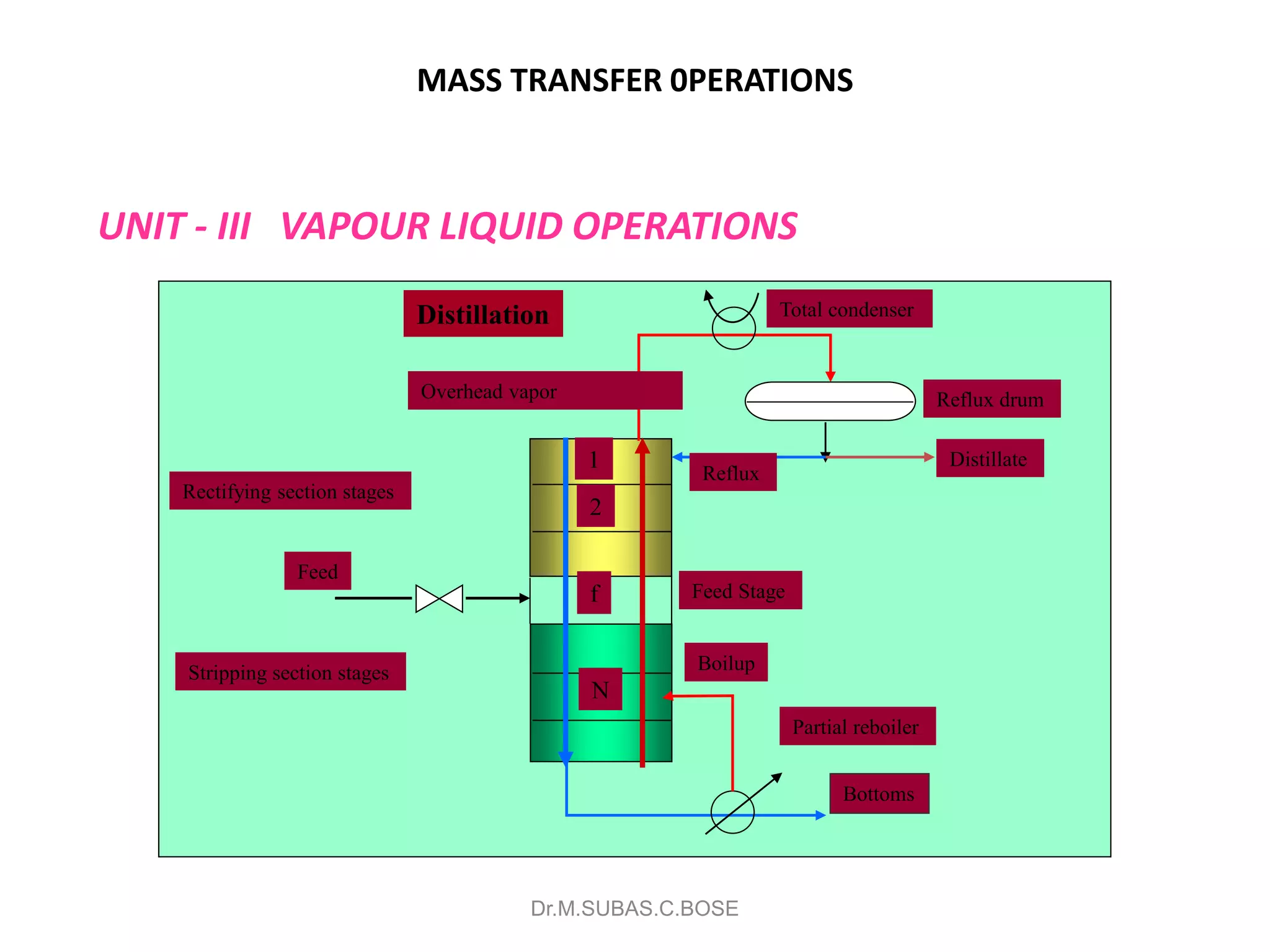
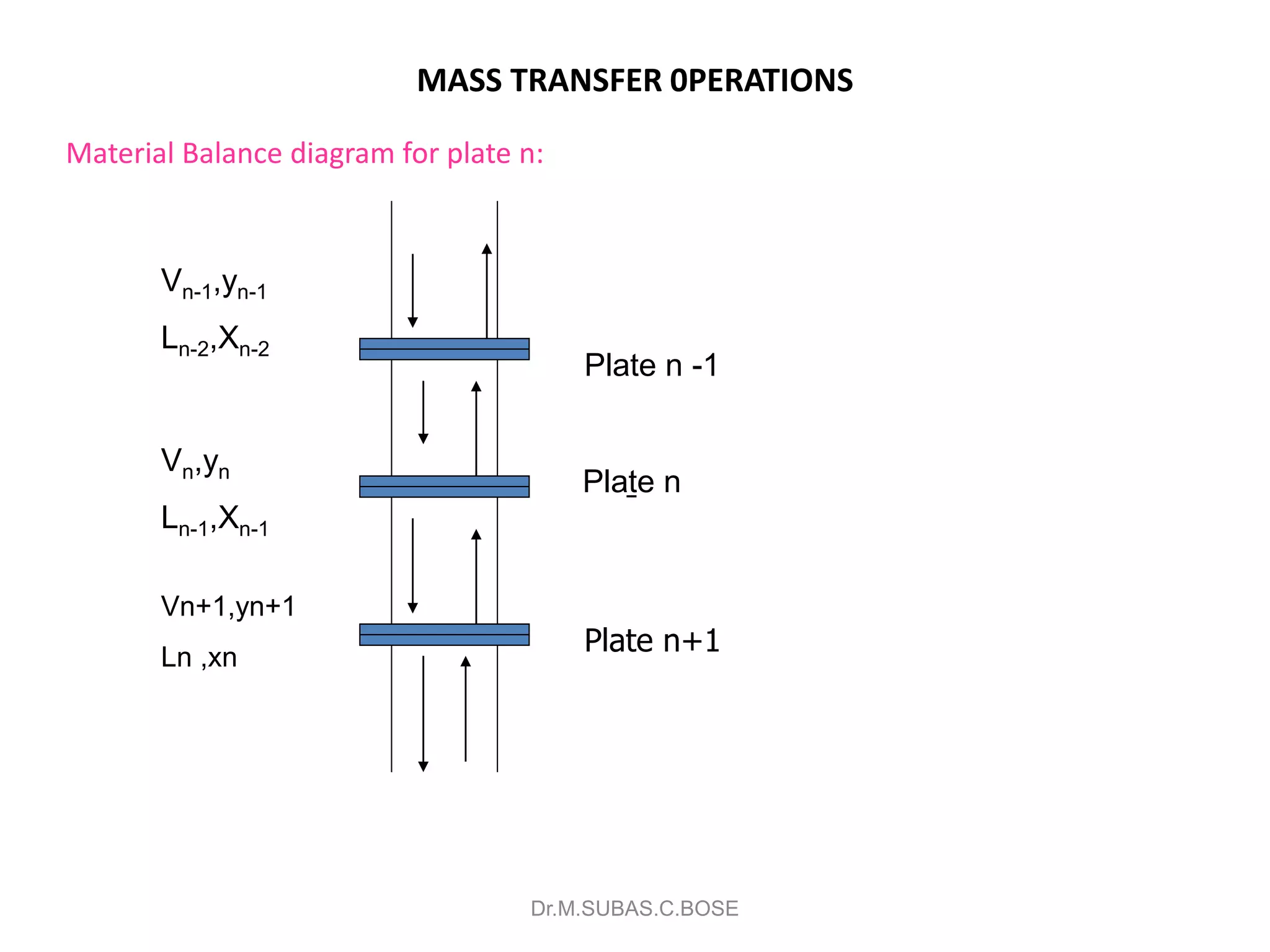
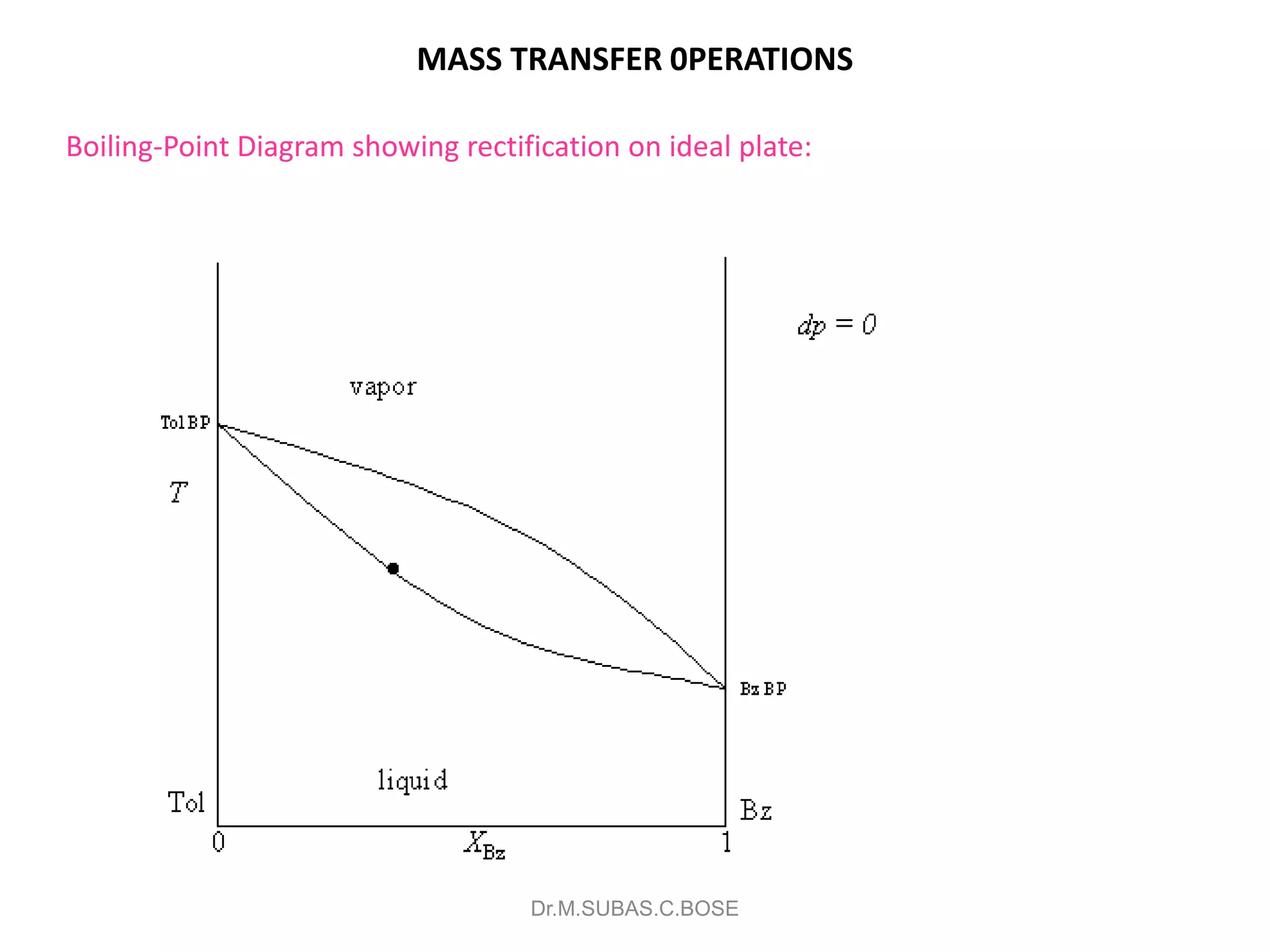
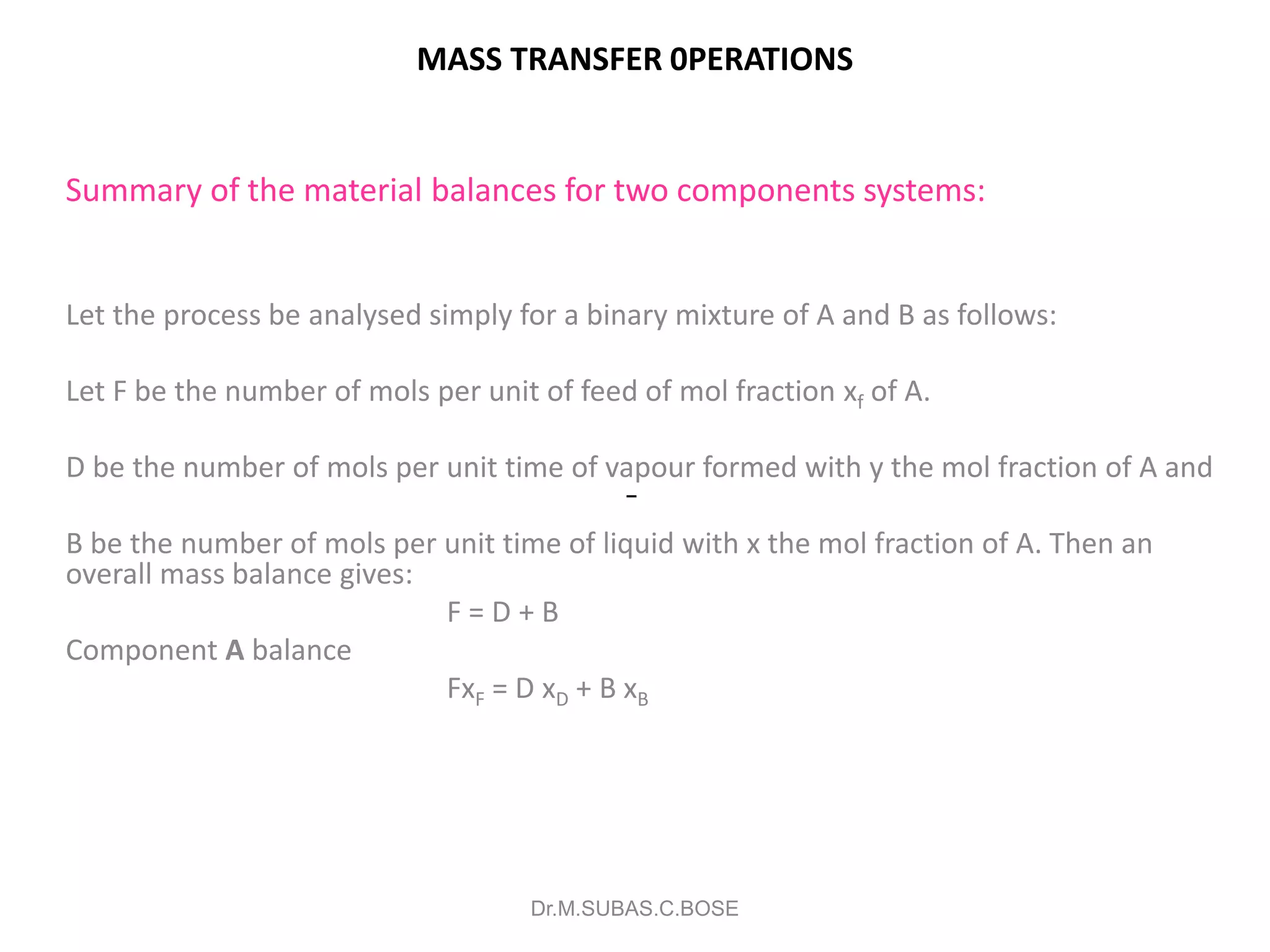
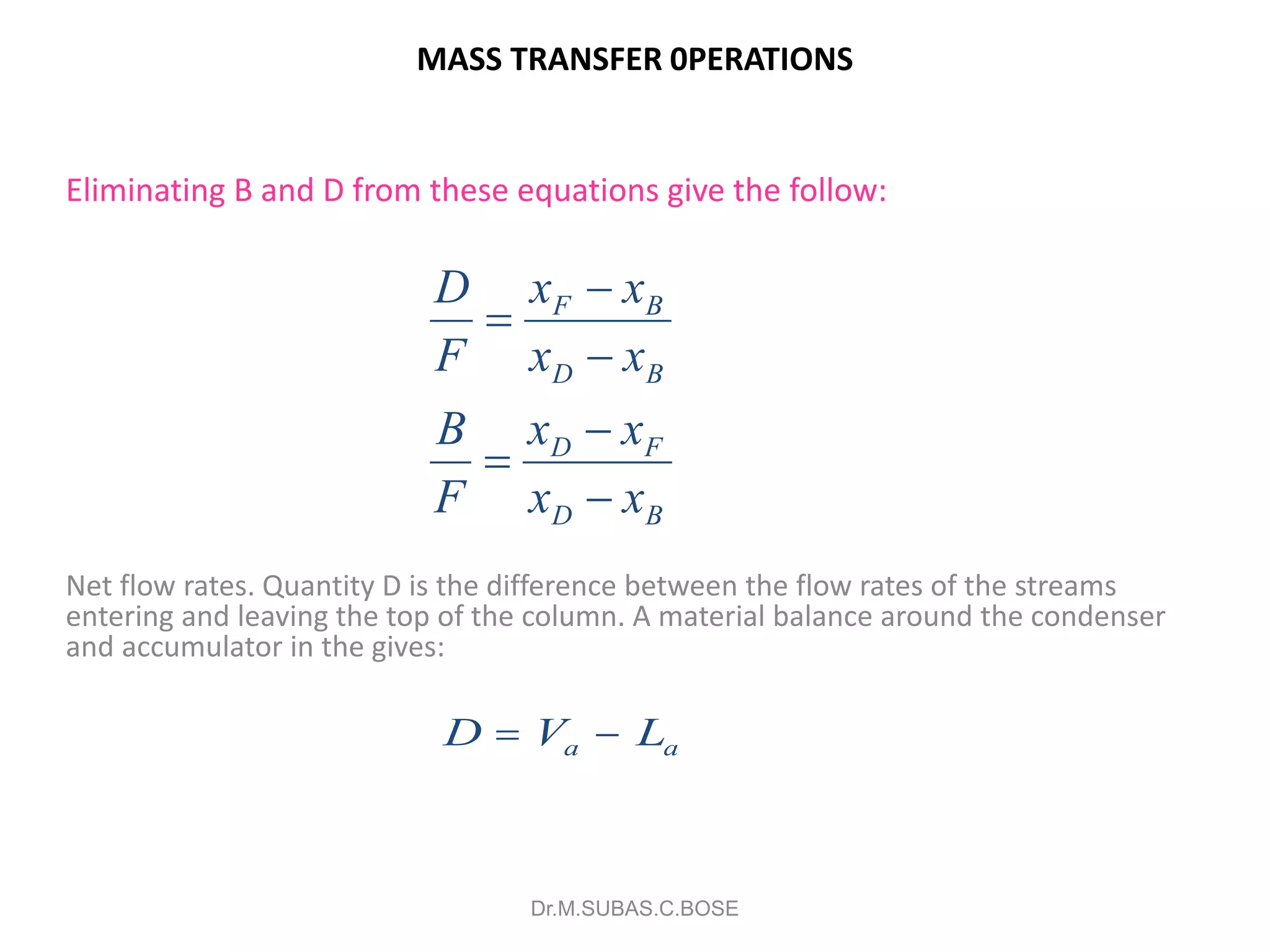
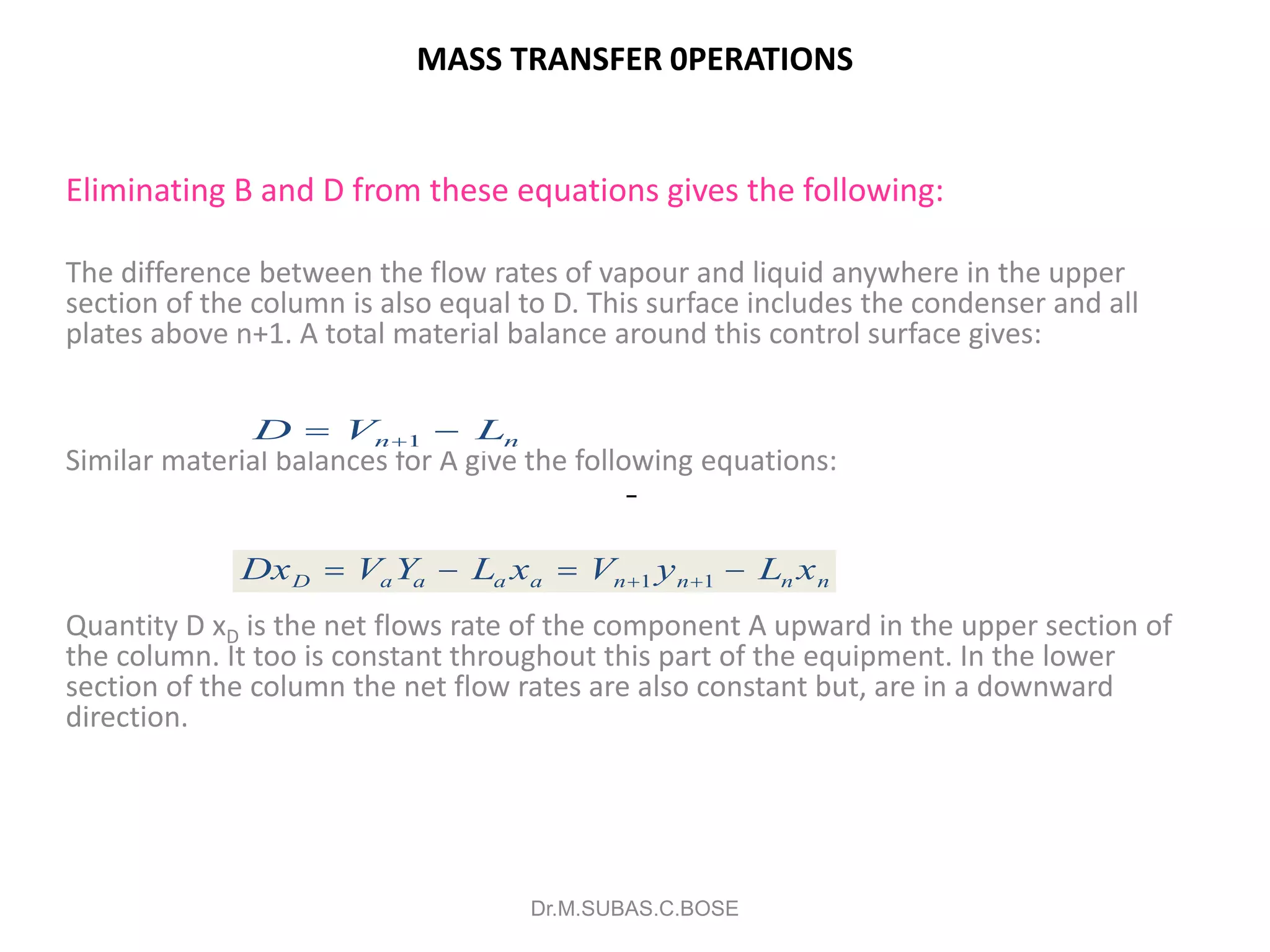
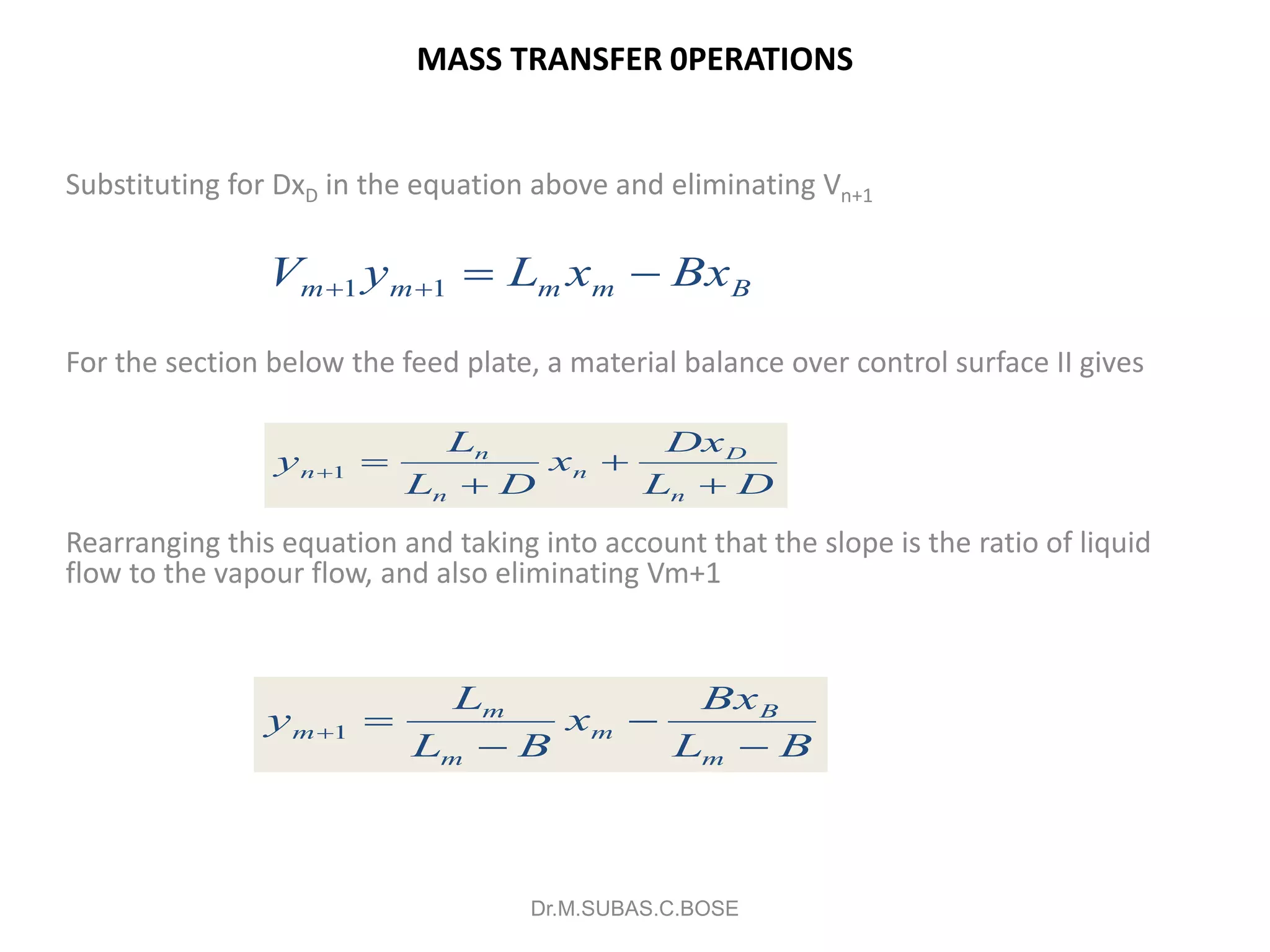
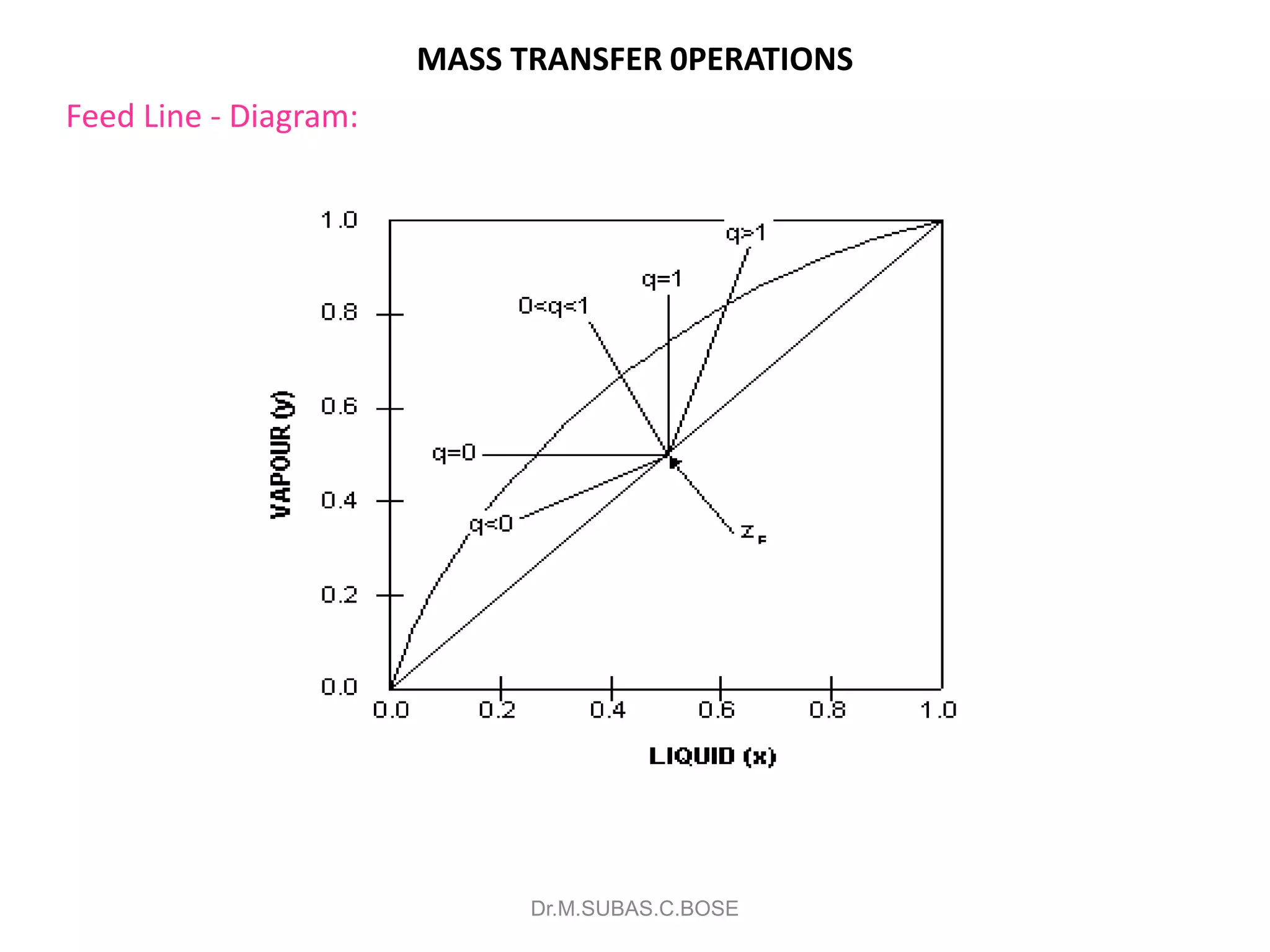
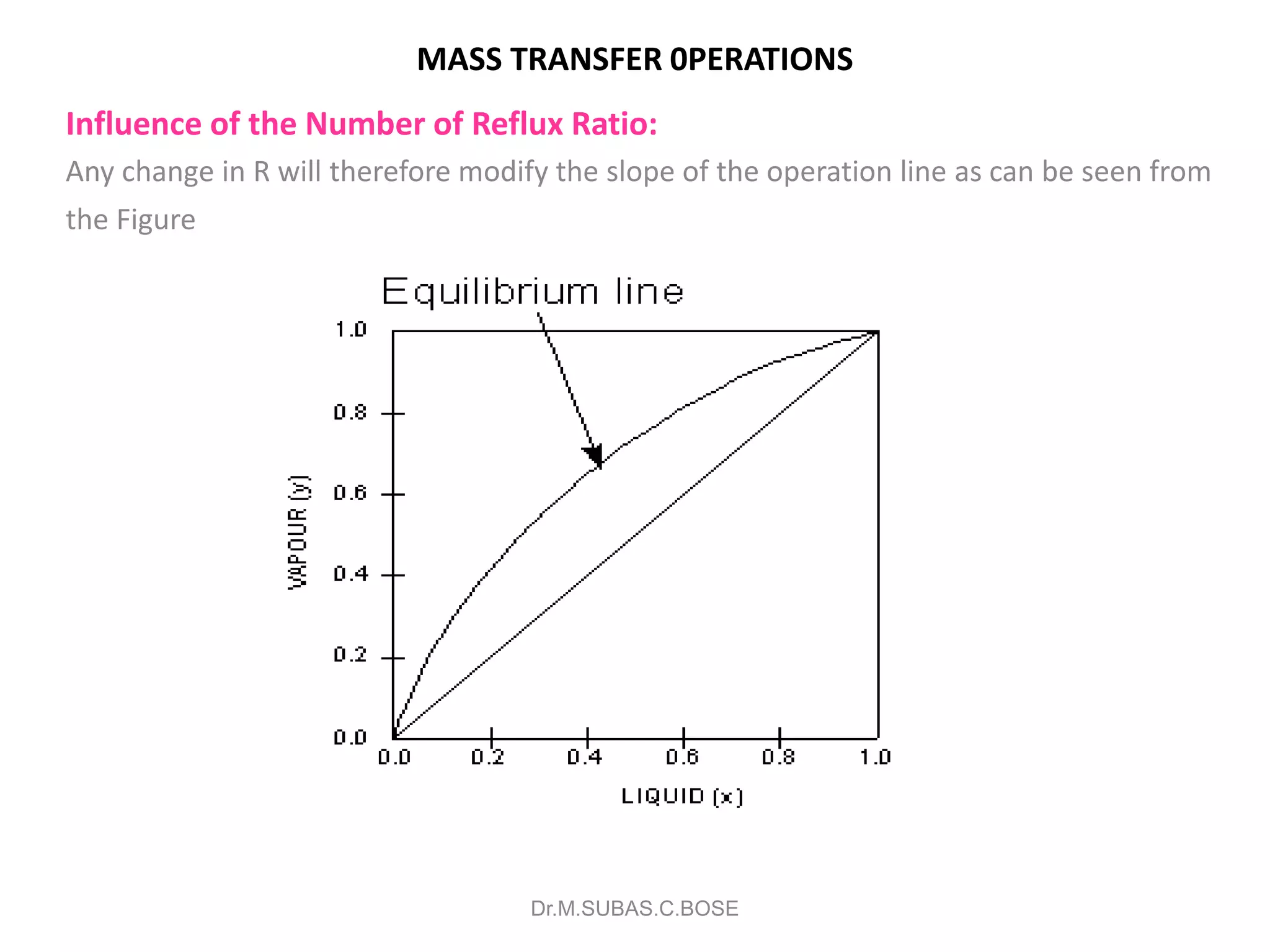
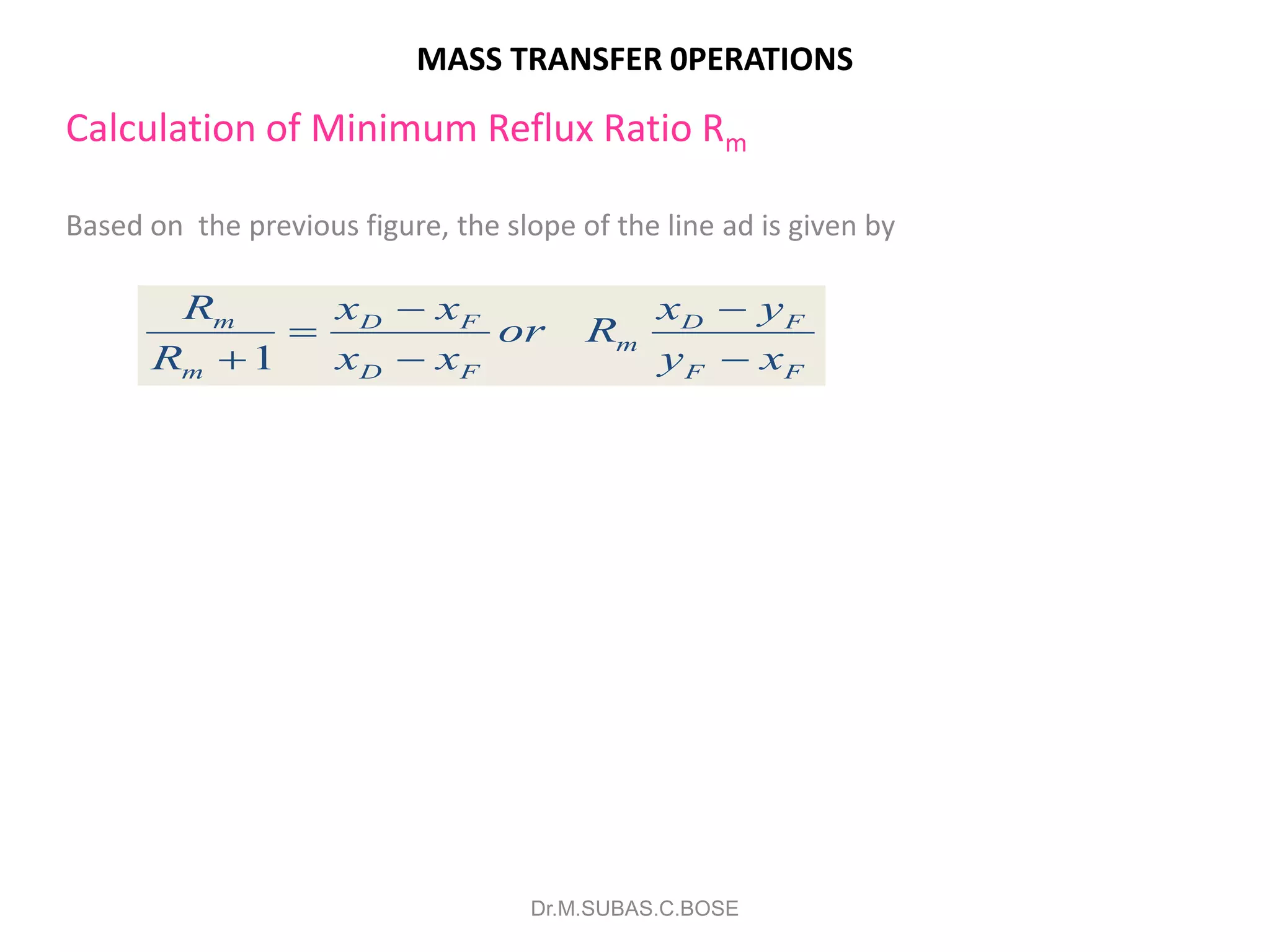
3) Distillation separates components based on volatility. Batch distillation methods include differential and flash distillation. Continuous rectification in distillation columns can be modeled using material balances