IB Chemistry on Dynamic Equilibrium and Equilibrium Constant
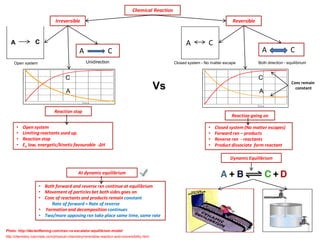
- 1. Dynamic Equilibrium Chemical Reaction Reversible Irreversible A C •Open system •Limiting reactants used up. •Reaction stop •Ea low, energetic/kinetic favourable -ΔH C A C •Closed system (No matter escapes) •Forward rxn – products •Reverse rxn - reactants •Product dissociate form reactant C Reaction going on Reaction stop Open system Unidirection A C Closed system - No matter escape Both direction - equilibrium A C •Both forward and reverse rxn continue at equilibrium •Movement of particles bet both sides goes on •Conc of reactants and products remain constant Rate of forward = Rate of reverse • Formation and decomposition continues •Two/more opposing rxn take place same time, same rate At dynamic equilibrium Conc remain constant Vs A A Photo: http://declanfleming.com/man-vs-escalator-equilibrium-model/ http://chemistry.tutorvista.com/physical-chemistry/reversible-reaction-and-irreversibility.html
- 2. Dynamic Equilibrium Closed system Reversible Forward Rate, Kf Reverse Rate, Kr Liquid -Vapour equilibrium Br2(l) ↔ Br2(g) initial equilibrium • Liq and gas Br2 in dynamic equilibrium • Add more liq Br2 will increase its liq mass but not conc • Dynamic equilibrium, Kc bet liq and gas Br2 remain the same • Macroscopic level – colour/intensity liq/gas Br2 remain constant • Microscopic level – liq/gas Br2 equilibrium, forward/ reverse rxn going on (Rate of Vapourization = Rate of Condensation) NO change in conc liquid/vapour Rate of evaporation = Rate of condensation Rate of evaporation > Rate of condensation More vapour form Rate condensation increase Initially Br2 (l) Br2(g) time Rate Rate of condensation Rate of evaporation Why add more liq Br2 will not change intensity vapour? Remove Br2 gas - Conc Br2 gas change - affect Kc (Rate of Vapourization > Rate of Condensation) Density = Mass Vol Conc = Mass Vol More mass - more vol Density/conc still same Rate of vapourization/condensation depend on change in conc Br2 (Rate of Vapourization = Rate of Condensation) No change in conc/intensity vapour Br2 Add more Br2
- 3. Dynamic Equilibrium Closed system Reversible Forward Rate, Kf Reverse Rate, Kr initial equilibrium NO change in conc sugar sol Rate of dissolving = Rate of crystallization Rate of dissolving > Rate of crystallization More sugar dissolve - saturated sol form Rate crystallization increase Initially time Rate Rate of crystallization Rate of dissolving Why add more sugar will not change sweetness/conc? Solute-solution equilibrium Sugar(s) ↔ Sugar (aq) • Sugar crystals/solution in dynamic equilibrium • Add sugar will not increase sugar conc/sweetness (saturated sol) • Dynamic equilibrium, Kc bet sugar solid and sol remain same • Macroscopic level – conc/sweetness remain constant • Microscopic level – crystal/sol in equilibrium, forward/reverse rxn going on (Rate of Dissolving = Rate of Crystallization) Adding more water – affect Kc – Conc sugar changes ( Rate of Dissolving > Rate of Crystallization ) Sugar (s) Sugar (aq) Add more sugar More mass - more vol Density/conc still same Conc = Mass Vol Density = Mass Vol Rate of dissolving/crystallization depend on change in sugar conc (Rate of Dissolving = Rate of Crystallization) No change in sugar conc (solution)
- 4. Dynamic Equilibrium Closed system Reversible Forward Rate, Kf Reverse Rate, Kr initial equilibrium NO change in conc vapour Rate of vapourization = Rate of crystallization Rate of vapourization > Rate of crystallization More iodine sublime Rate crystallization increase Initially time Rate Rate of crystallization Rate of vapourization Why add more I2 will not change vapour pressure/intensity? Solid-vapour equilibrium Iodine(s) ↔ Vapour(g) • I2 solid/vapour in dynamic equilibrium • Add more I2 will not increase vapour pressure I2 • Equilibrium, Kc bet solid/vapour remain the same (Temp dependent) • Macroscopic level – Vapour pressure/intensity remain constant • Microscopic level – solid/vapour in equilibrium, forward/reverse rxn going on (Rate of Vapourization = Rate of Crystallization) Using a bigger container. Will vapour pressure change? Iodine (s) Iodine (g) Add more I2 More mass - more vol Density/conc still same Conc = Mass Vol Density = Mass Vol Rate of vapourization/crystallization depend on change in conc I2 (Temp dependent) (Rate of Vapourization = Rate of Crystallization) Vapour pressure same
- 5. Dynamic Equilibrium Closed system Reversible Forward Rate, Kf Reverse Rate, Kr Liquid -Vapour equilibrium Br2(l) ↔ Br2(g) initial equilibrium NO change in conc liquid/intensity vapour/vapour pressure Rate of evaporation = Rate of condensation Liquid Br2 evaporate Macroscopic – no changes 2NO2(g) N2O4(g) Physical system Chemical system Vapour Br2 condense Forward rate rxn Rate Combining Backward rate rxn Rate decomposition Reversible rxn happening, same time with same rate Rate of forward = Rate of backward Conc of reactants and products remain UNCHANGED not EQUAL combining decomposition brown colourless
- 6. Dynamic Equilibrium Closed system Reversible Forward Rate, Kf Reverse Rate, Kr 2NO2(g) N2O4(g) Chemical system Forward rate rxn Rate Combining Backward rate rxn Rate dissociation Reversible rxn happening, same time with same rate Rate of forward = Rate of backward Conc of reactant and product remain UNCHANGED/CONSTANT not EQUAL combining dissociation Conc vs time Rate vs time Conc Time Conc NO2 Conc N2O4 With time •Conc NO2 decrease ↓ - Forward rate decrease ↓ •Conc N2O4 increase ↑ - Backward rate increase ↑ 2NO2(g) N2O4(g) Forward rate Backward rate Forward Rate = Backward Rate Conc NO2 and N2O4 remain UNCHANGED/CONSTANT brown colourless
- 7. How dynamic equilibrium is achieved in closed system? Conc of NO2 decrease ↓over time Forward rate, Kf decrease ↓ over time Forward Rate = Reverse Rate NO2 2NO2(g) N2O4(g) Conc of N2O4 increase ↑ over time N2O4 Reverse rate, Kr increase ↑ over time NO2 N2O4 1 2 Conc of reactant/product remain constant Rate 3 Time Conc NO2 N2O4 At dynamic equilibrium As reaction proceeds concentration As reaction proceeds rate Time
- 8. Dynamic Equilibrium Reversible (closed system) Forward Rate, K1 Reverse Rate, K-1 Kc = ratio of molar conc of product (raised to power of their respective stoichiometry coefficient) to molar conc of reactant (raised to power of their respective stoichiometry coefficient) Conc of product and reactant at equilibrium At Equilibrium Forward rate = Backward rate Conc reactants and products remain CONSTANT/UNCHANGE Equilibrium Constant Kc aA(aq) + bB(aq) cC(aq) + dD(aq) coefficient Solid/liq not included in Kc Conc represented by [ ] K1 K-1 a b c d c A B C D K 1 1 K K Kc Equilibrium Constant Kc express in Conc vs time Rate vs time A + B C + D Conc Time Click here notes on dynamic equilibrium Excellent Notes K1 = forward rate constant K-1 = reverse rate constant
- 9. Large Kc • Position equilibrium shift to right • More product > reactant Magnitude of Kc a b c d c A B C D K Extend of reaction How far rxn shift to right or left? Not how fast a b c d c A B C D K Small Kc • Position equilibrium shift to left • More reactant > product c K c K Position of equilibrium 2CO2(g) ↔ 2CO(g) + O2(g) 92 3 10 c K 2H2(g) + O2(g) ↔ 2H2O(g) 81 310 c K H2(g) + I2(g) ↔ 2HI(g) 2 8.710 c K 1 Moderate Kc • Position equilibrium lies slightly right • Reactant and product equal amount Reaction completion Reactant favoured Reactant/Product equal Product favoured c K Temp dependent Extend of rxn Not how fast
- 10. Equilibrium Constant Kc a b c d c A B C D K aA(aq) + bB(aq) cC(aq) + dD(aq) Conc of product and reactant at equilibrium Equilibrium expression HOMOGENEOUS gaseous rxn 4NH3(g) + 5O2(g) ↔ 4NO(g) + 6H2O(g) N2(g) + 3H2(g) ↔ 2NH3(g) NH4CI(s) ↔ NH3(g) + HCI(g) 2SO2(g) + O2(g) ↔ 2SO3(g) 5 2 4 3 6 2 4 NH O NO H O Kc 3 2 1 2 2 3 N H NH Kc 1 1 3 K NH HCI c 0 4 1 1 3 NH CI NH HCI Kc 1 2 2 2 2 3 SO O SO Kc Equilibrium expression HETEROGENOUS rxn CaCO3(s) ↔ CaO(g) + CO2(g) 0 3 1 2 1 CaCO CaO CO Kc 1 2 1 K CaO CO c CH3COOH(l) + C2H5OH(l) ↔ CH3COOC2H5(l) + H2O(l) 1 2 5 1 3 1 2 1 3 2 5 CH COOH C H OH CH COOC H H O Kc Equilibrium expression HOMOGENEOUS liquid rxn Cu2+ (aq) + 4NH3(aq) ↔ [Cu(NH3)4]2+ 4 3 2 1 2 3 4 ( ) Cu NH Cu NH Kc Reactant/product same phase Reactant/product diff phase Solid and liq - conc no change (not included)
- 11. Conc vs Time How dynamic equilibrium is achieved in a closed system? 40 0 Rate forward = ½ breakdown = ½ x 40 = 20 Rate reverse = ¼ form = ¼ x 0 = 0 20 20 Rate forward = ½ breakdown = ½ x 20 = 10 Rate reverse = ¼ form = ¼ x 20 = 5 15 25 Rate forward = ½ breakdown = ½ x 15 = 8 Rate reverse = ¼ form = ¼ x 25 = 6 13 27 Rate forward = ½ breakdown = ½ x 13 = 7 Rate reverse = ¼ form = ¼ x 27 = 7 13 27 At dynamic Equilibrium Rate forward = Rate reverse Breakdown (7) = Formation (7) At dynamic Equilibrium Conc reactant 13 /Product 27 constant Rate vs Time 1/ 4 1/ 2 .. tan .. .. tan .. 1 1 rate cons t reverse rate cons t forward K K 2 13 27 tan reac t product Kc 2 1/ 4 1/ 2 1 1 K K Kc or
- 12. Click here to view simulation Click here simulation using paper clips Click here simulation on reversible rxn Click here on reversible rxn Simulation on Dynamic equilibrium Click here on equilibrium constant
