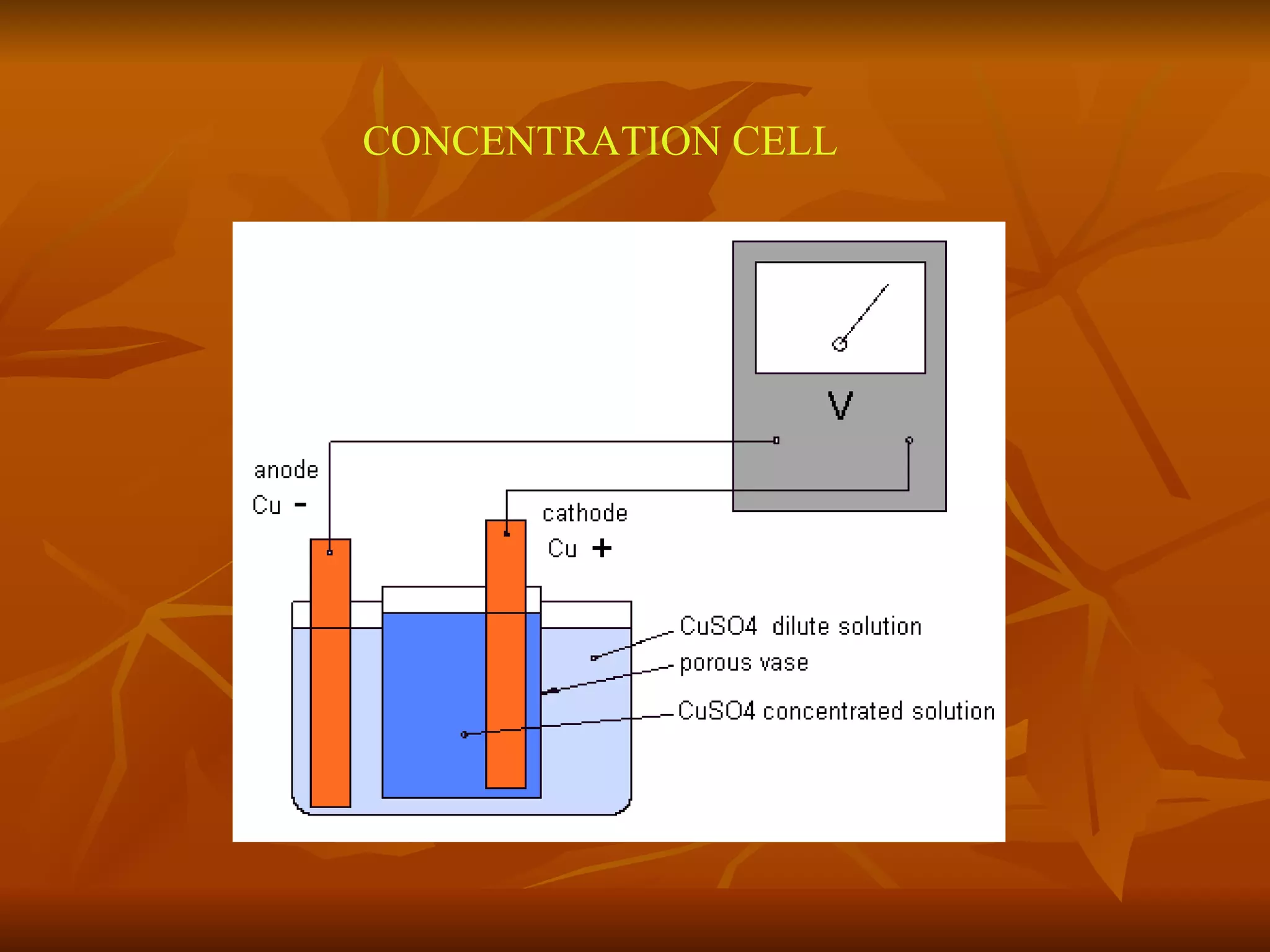
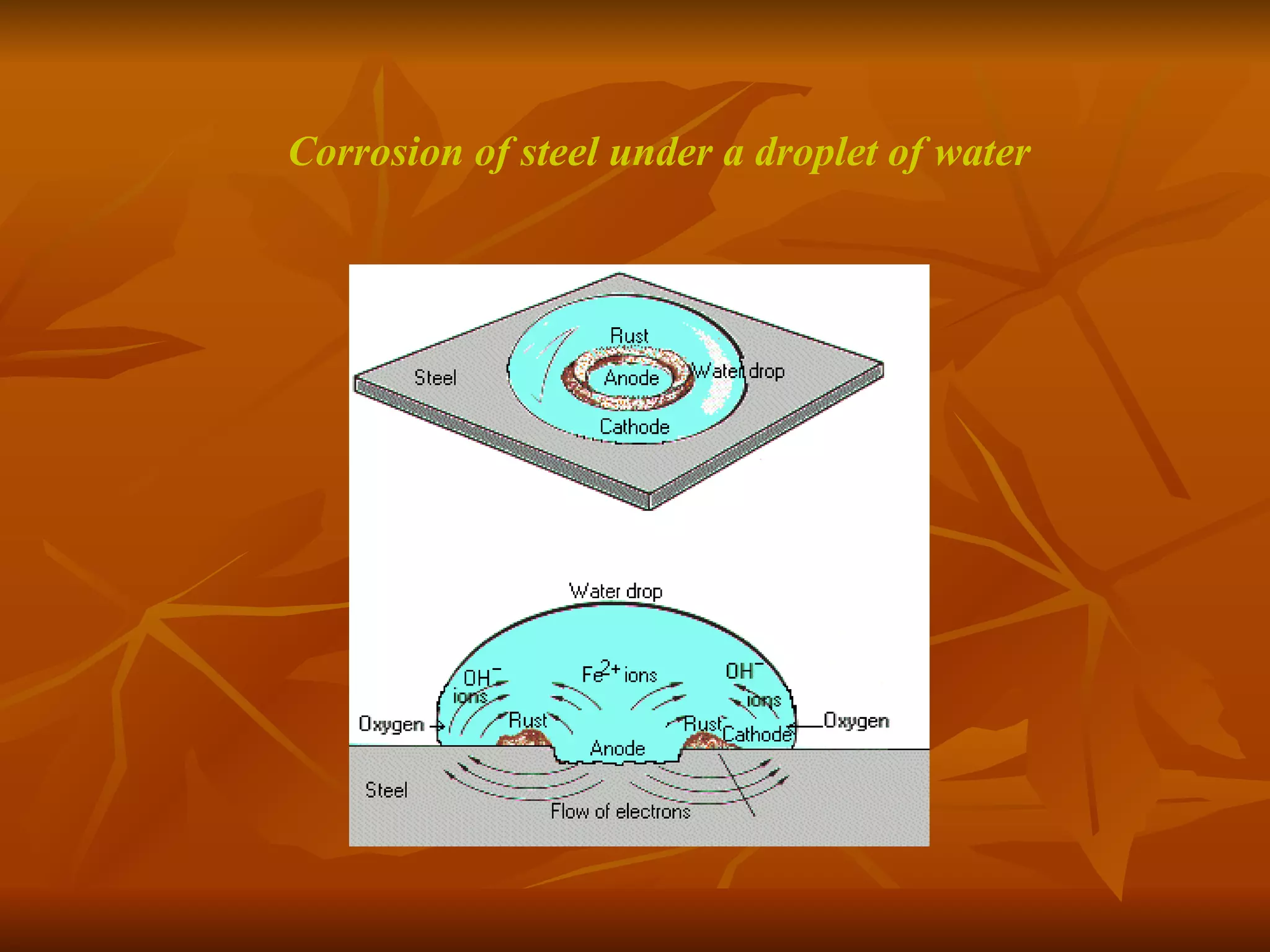
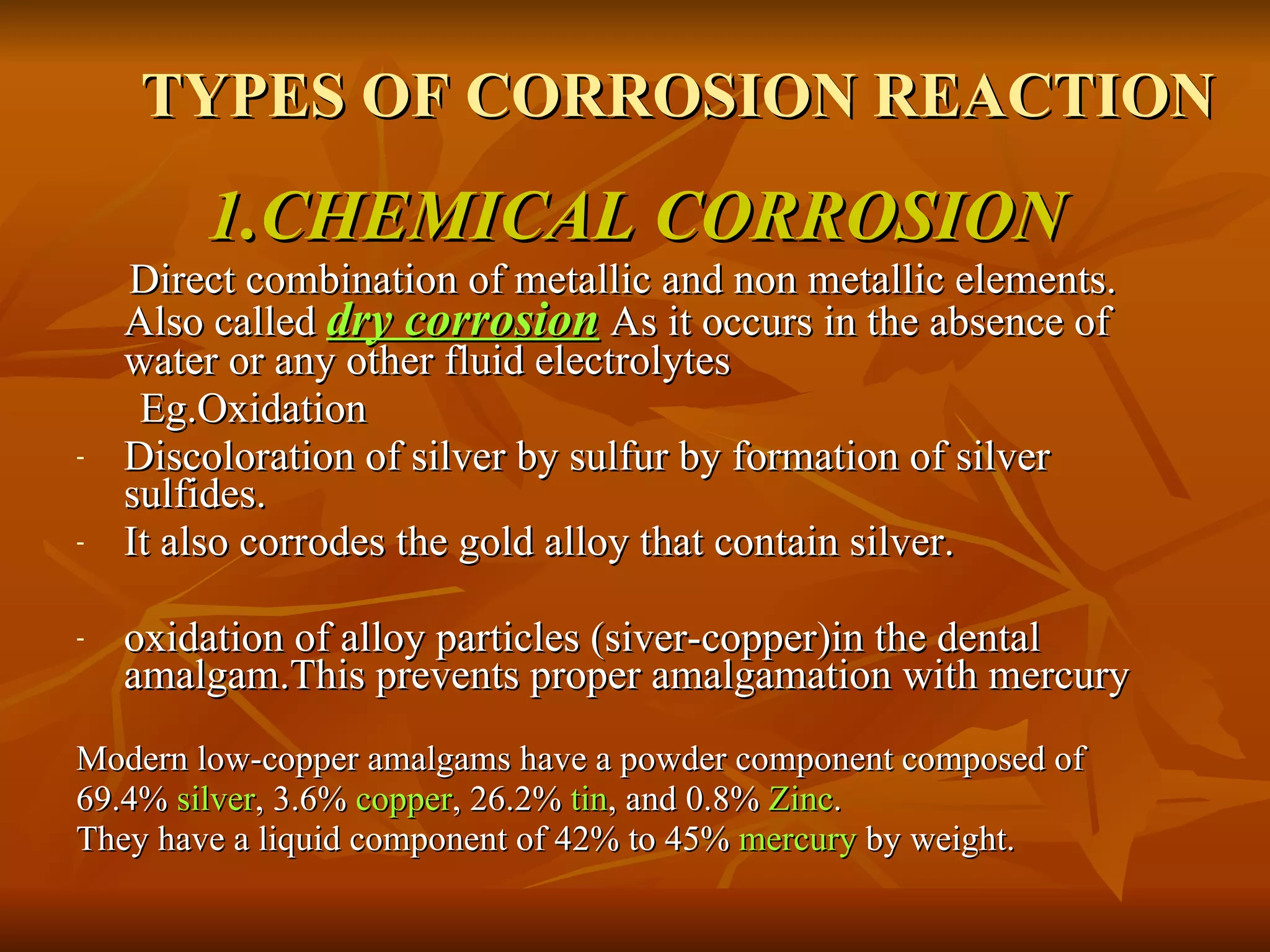
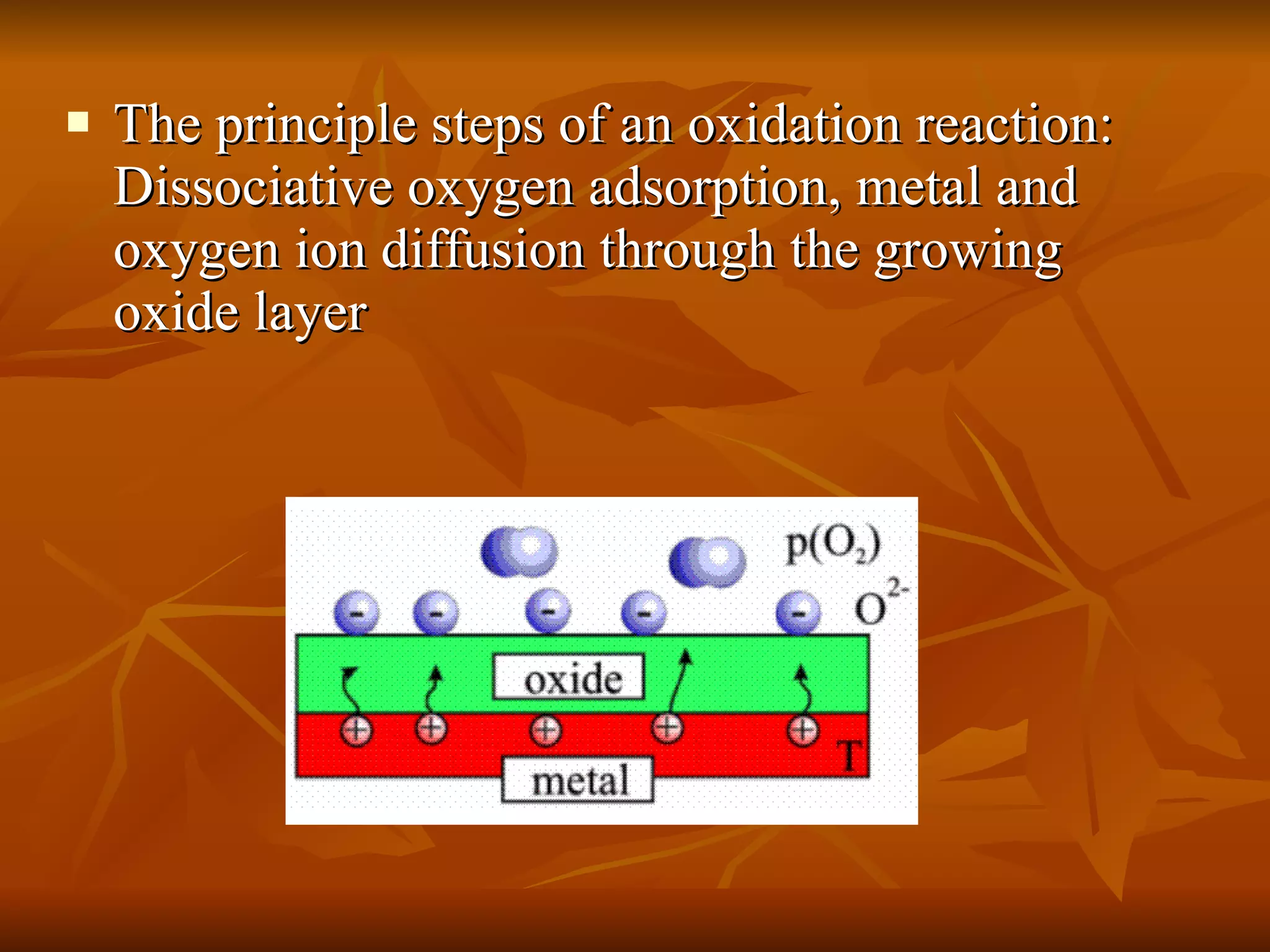
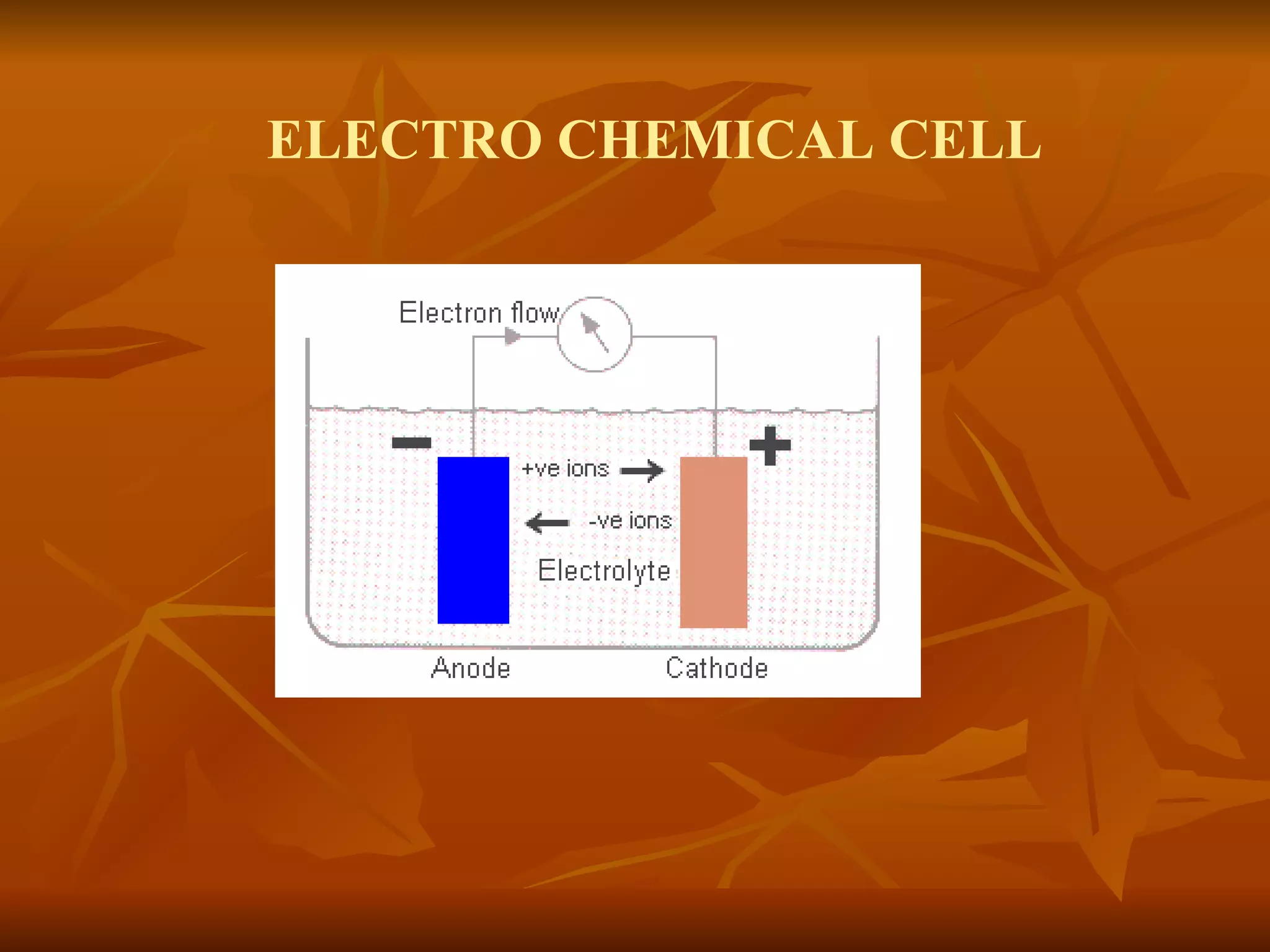
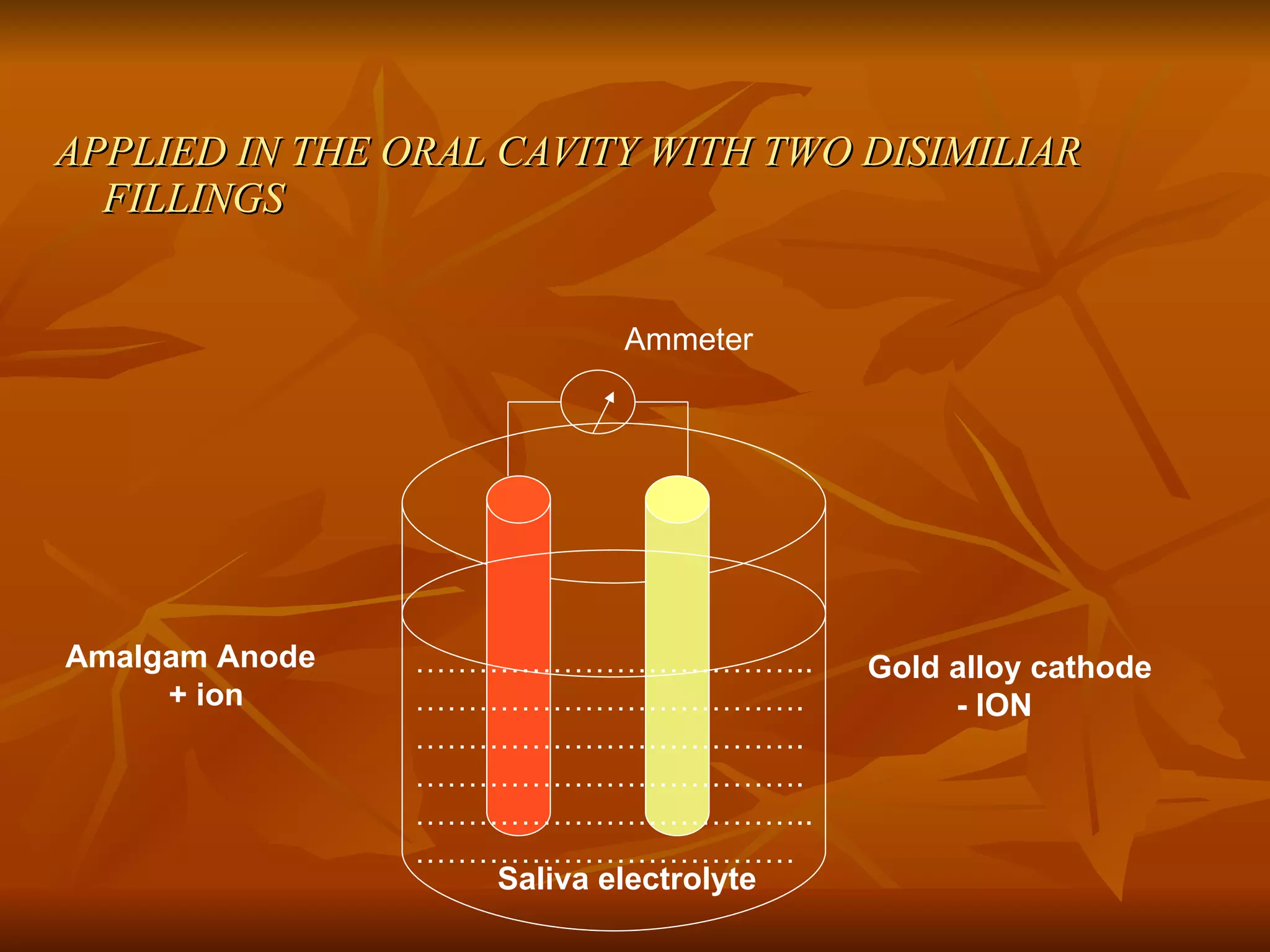
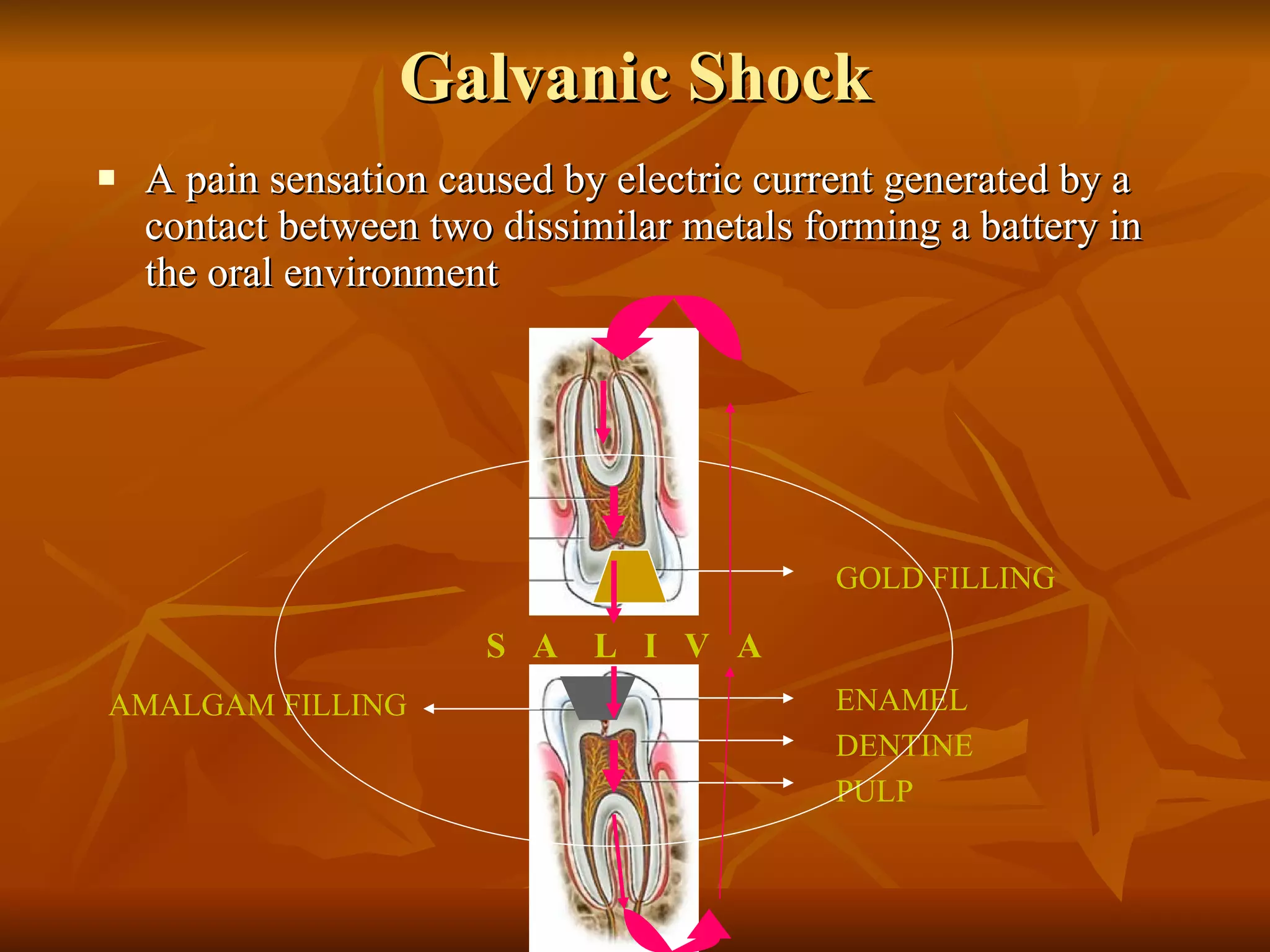
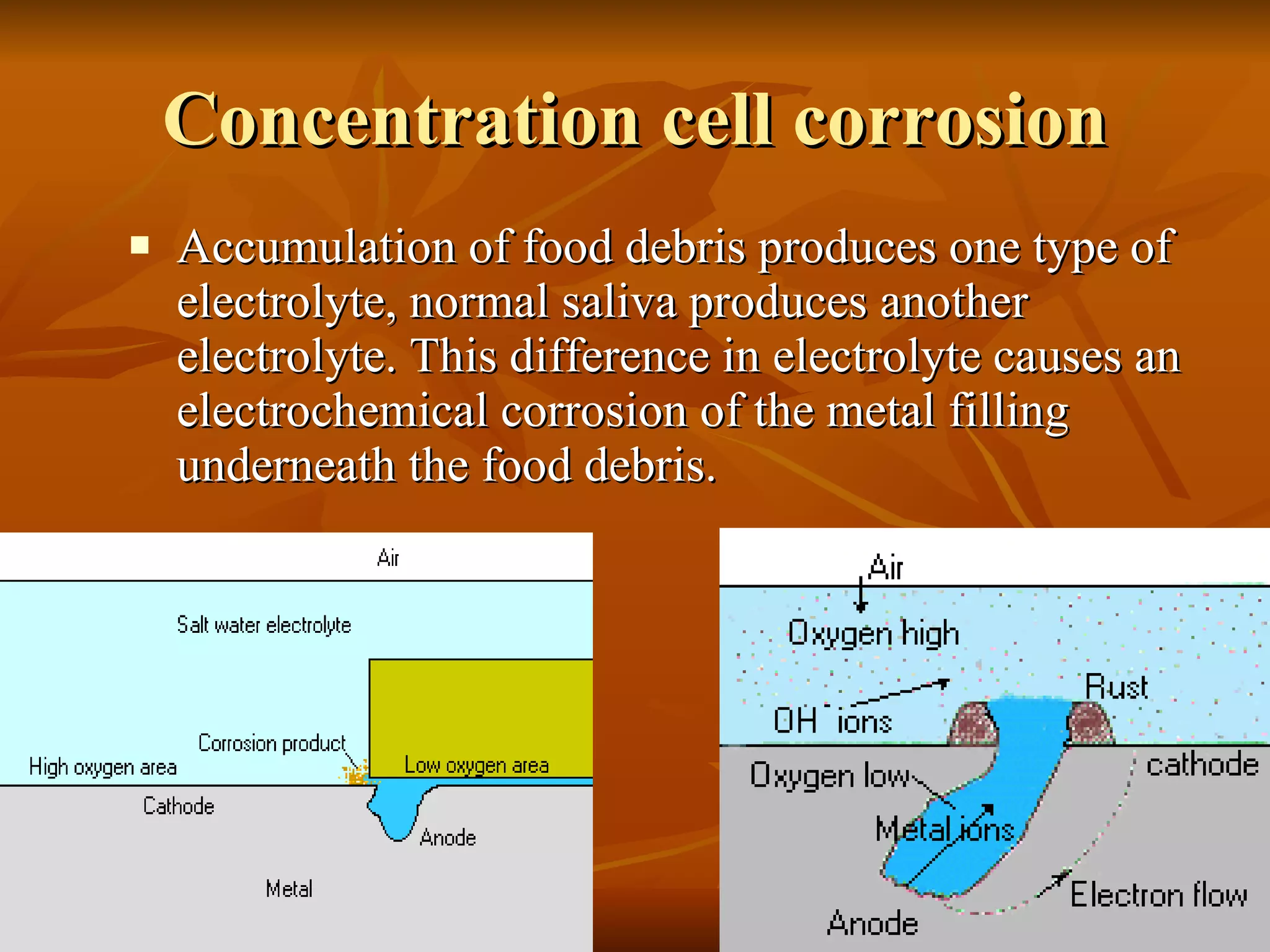
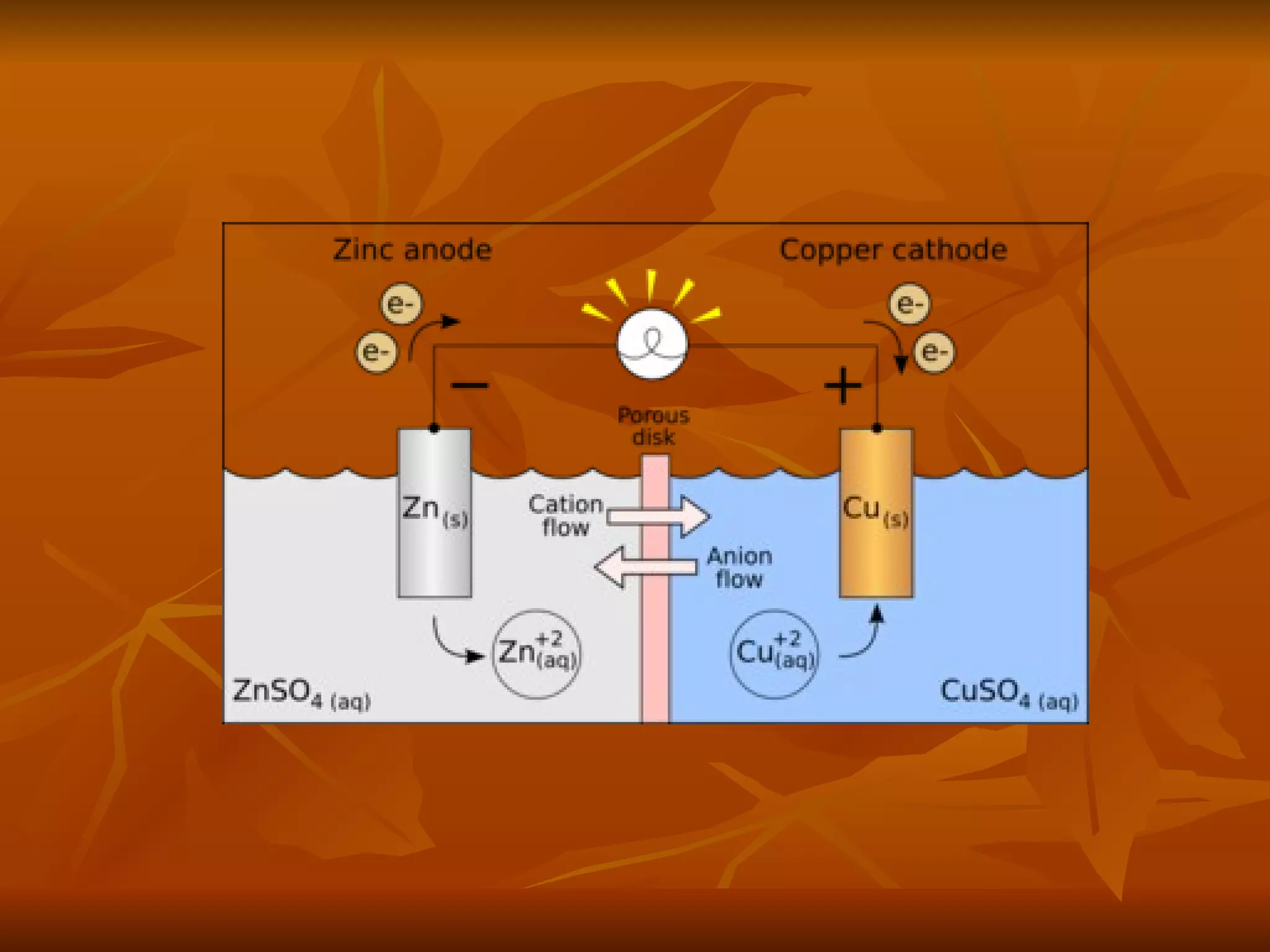
The document discusses various types of corrosion that can occur in dental materials including crevice corrosion, galvanic corrosion, pitting corrosion, stress corrosion, and concentration cell corrosion. It also discusses tarnish, which is the dulling or discoloration of metal surfaces through chemical film formation. Factors that can lead to corrosion and tarnish of dental restorations include dietary and oral hygiene habits, bacterial activity, and presence of acids, chlorides, and other chemicals in the oral environment. Protection against corrosion involves use of alloys with noble metals, polishing of surfaces, and application of protective coatings.