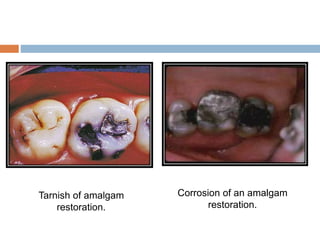
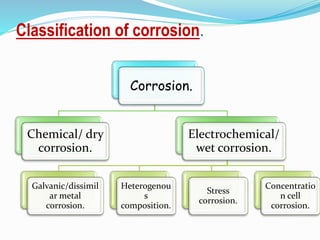
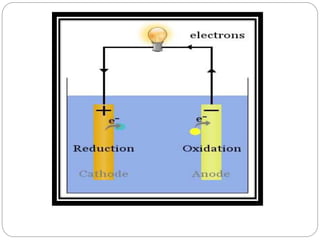
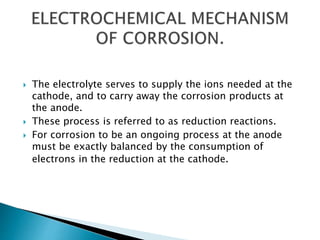
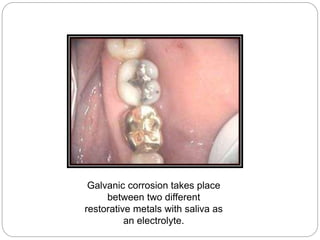
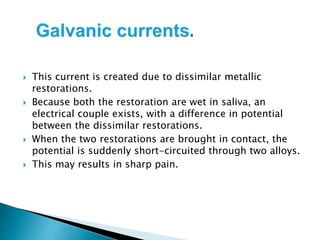
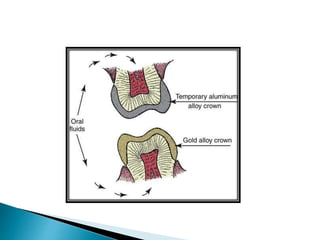
Tarnish and corrosion are natural processes that affect metals used in dentistry. Tarnish is a surface discoloration caused by thin oxide, sulfide, or chloride films, while corrosion deteriorates the actual metal through chemical or electrochemical reactions. Corrosion occurs due to metals striving for their lowest energy state and reacting with oxygen, sulfur, or chlorine. It can be protected against by passivating alloys, increasing their noble metal content, avoiding galvanic interactions between dissimilar metals, and maintaining a clean surface. Understanding and controlling corrosion is important for increasing the longevity of dental restorations.