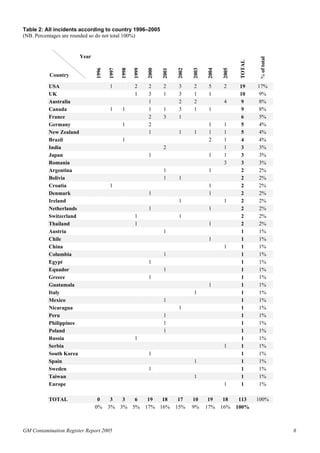
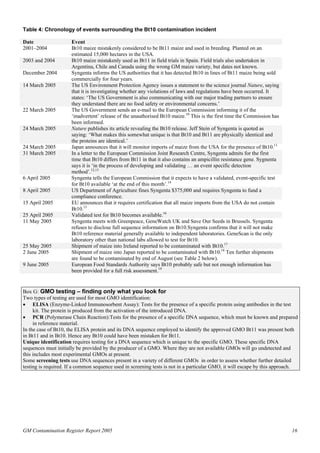
The 2005 GM Contamination Report by Genewatch UK and Greenpeace reviews contamination cases, illegal plantings, and negative agricultural effects related to genetically modified organisms (GMOs) from 1996 to 2005, with 113 incidents reported in total. The report highlights issues such as ineffective controls, illegal sales of GM crops, and the need for independent monitoring and transparency regarding GMO releases. Urgent recommendations include establishing an international commission for investigation and stricter regulations to prevent future contamination and protect public interests.