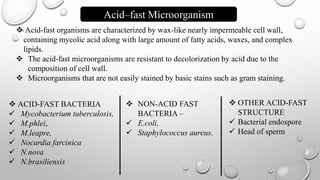
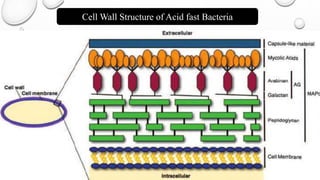
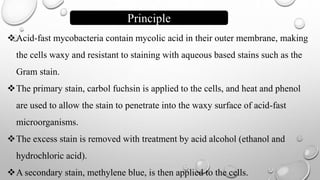
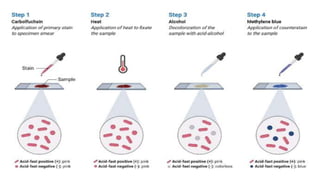
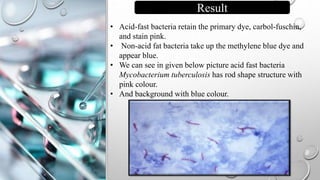
The document describes the Ziehl-Neelsen staining technique developed for the identification of acid-fast microorganisms, particularly Mycobacterium tuberculosis, by distinguishing them from non-acid fast bacteria. It details the procedure, principles, reagents, and applications of this staining method. Additionally, it highlights the significance of the structural composition of acid-fast bacteria and mentions potential modifications and applications in various microbiological examinations.