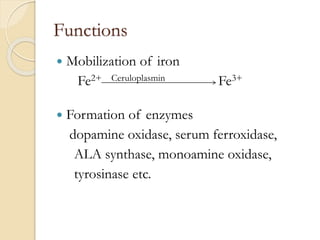
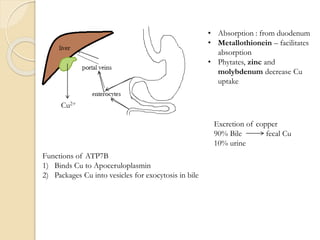
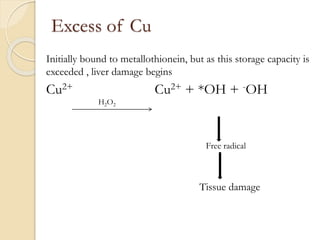
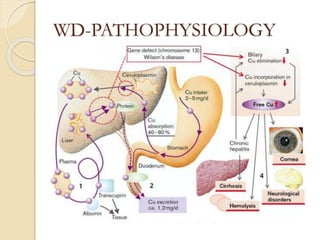
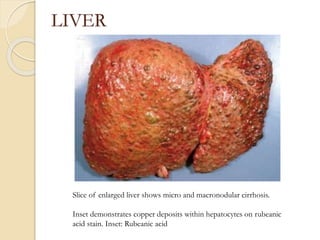
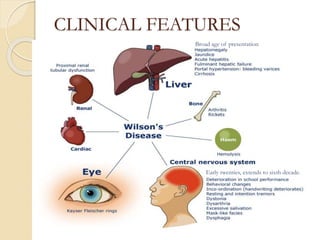
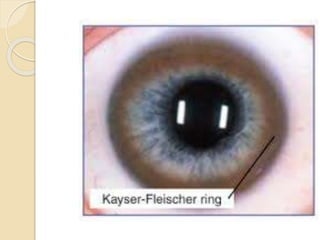
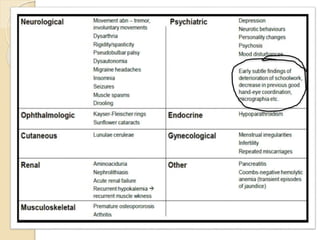
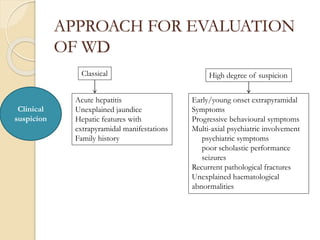
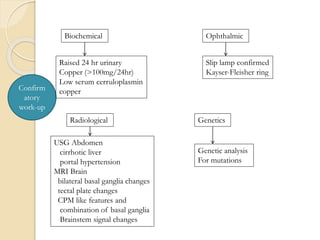
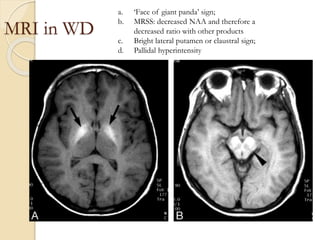
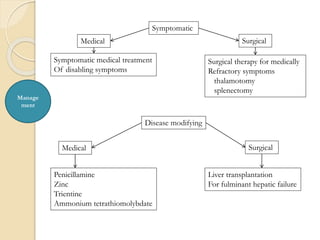
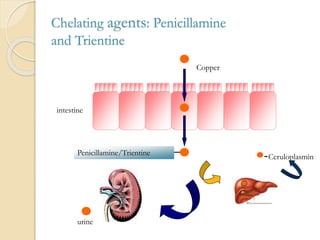
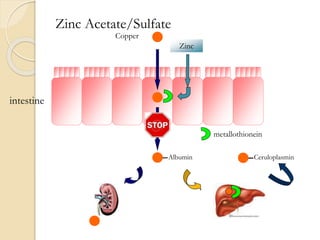
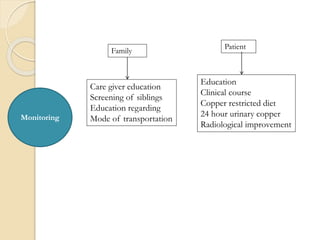
Wilson's disease is caused by a genetic defect resulting in excessive copper deposition in tissues. It impairs the liver's ability to excrete copper into bile. Clinical features vary widely and include hepatic, neurological or psychiatric symptoms. Diagnosis involves low serum ceruloplasmin, high urinary copper, and Kayser-Fleisher rings on eye exam. Treatment includes copper-restricting agents like penicillamine or trientine to increase copper excretion, or zinc to reduce copper absorption. Liver transplantation may be considered for severe liver disease. With lifelong treatment, prognosis is good if Wilson's disease is diagnosed and managed early.