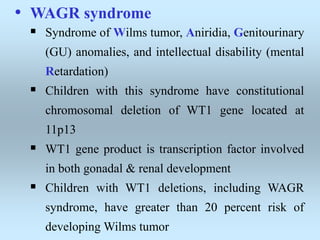
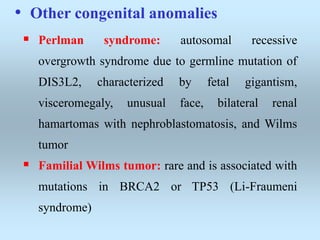
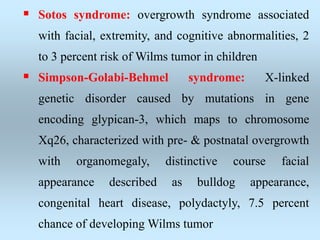
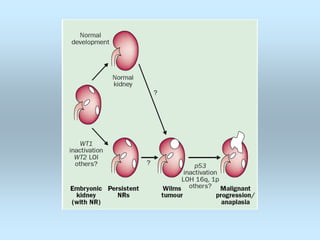
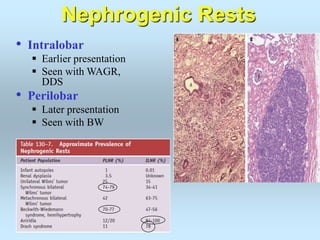
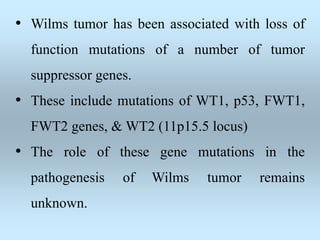
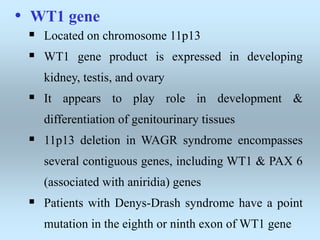
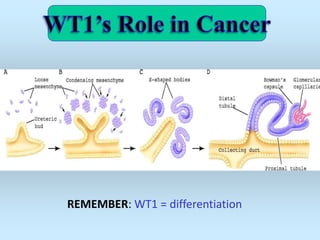
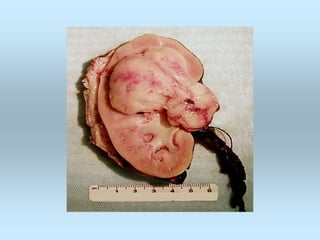
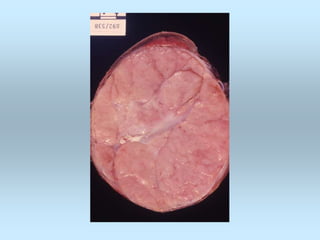
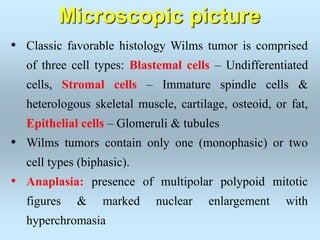
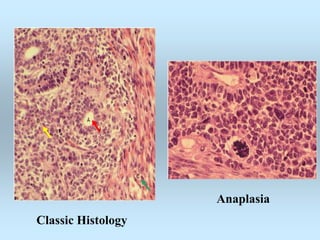
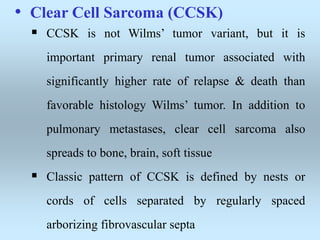
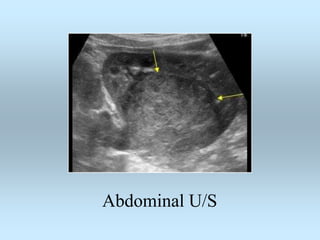
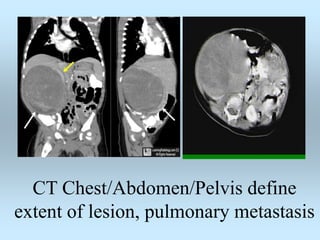
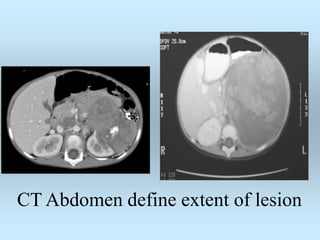
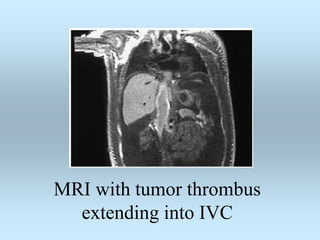
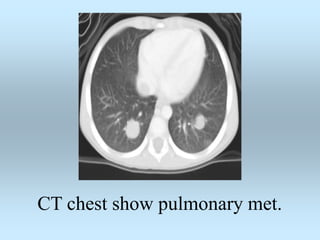
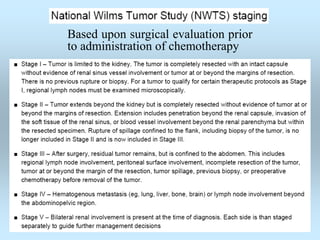
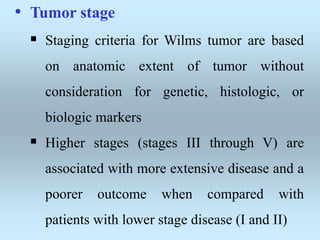
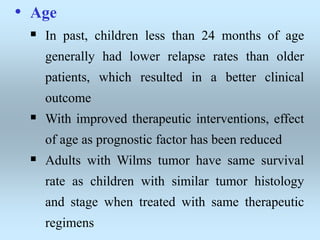
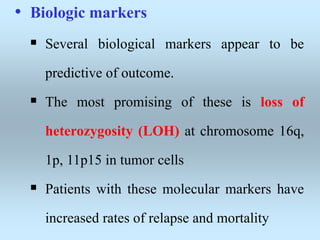
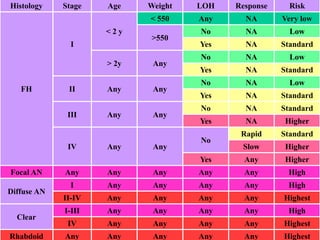
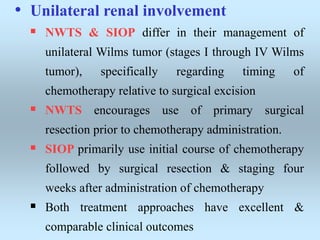
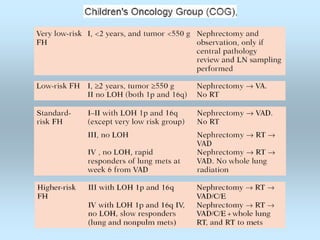
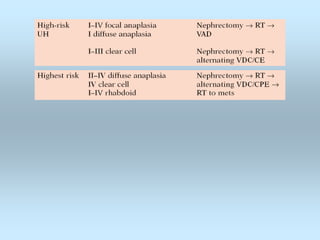
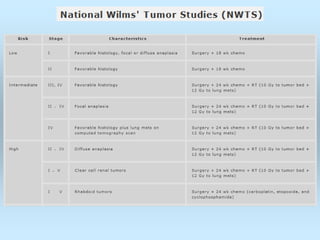
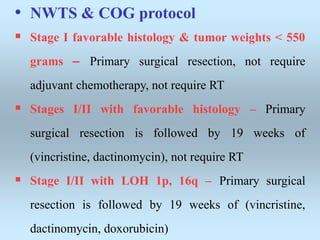
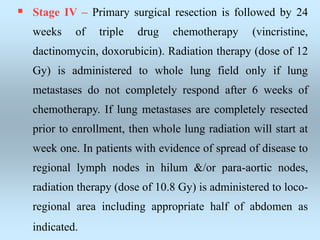
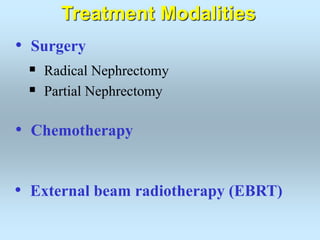
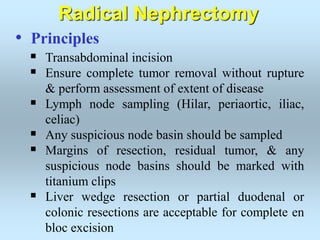
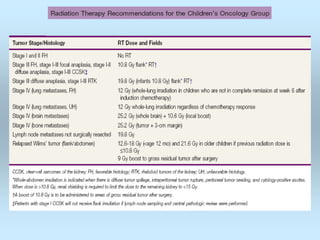
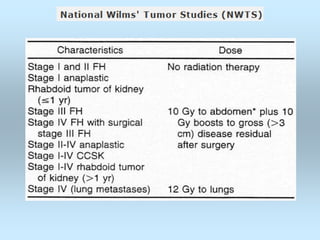
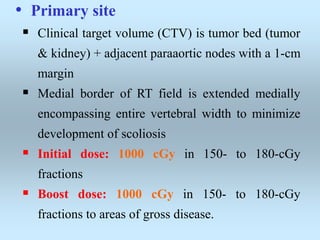
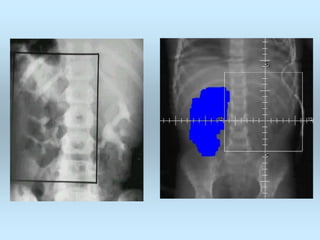
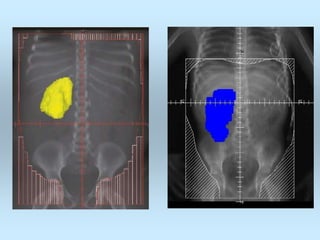
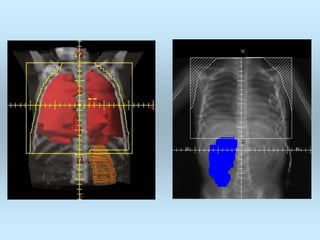
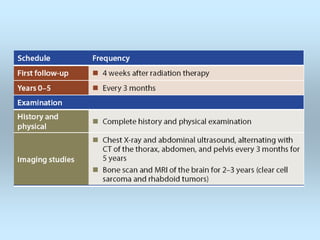
Wilm's tumor is the most common renal malignancy in children. It typically presents as a painless abdominal mass in children around 3 years old. Imaging such as ultrasound and CT are used to evaluate the tumor extent and presence of metastases. The tumor is staged surgically, with higher stages indicating a worse prognosis. Histology is also important, as the presence of anaplasia predicts a poorer outcome. Genetic conditions like WAGR and Denys-Drash syndromes increase the risk of developing Wilm's tumor. Treatment involves surgery, chemotherapy, and sometimes radiation therapy.