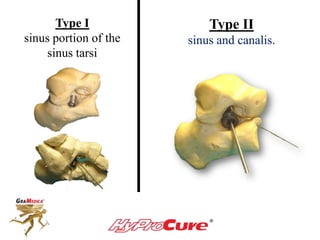
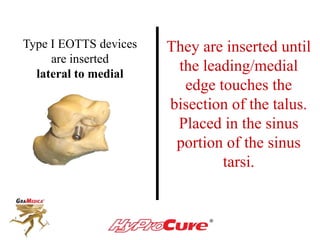
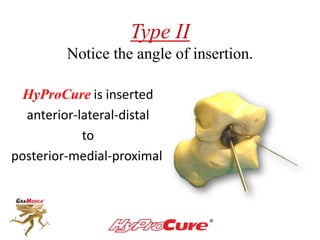
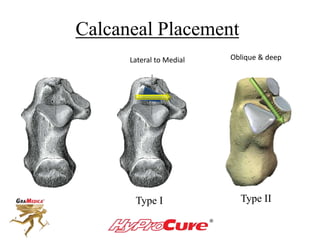
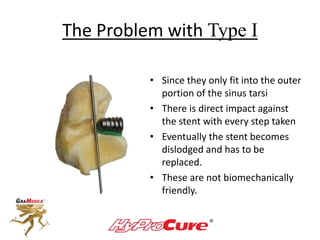
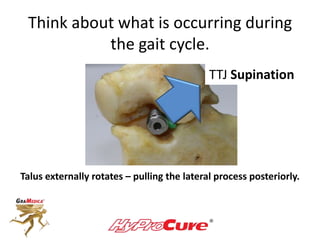
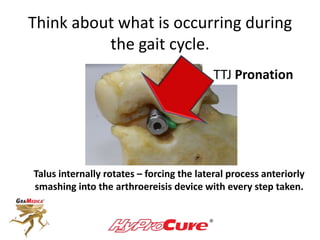
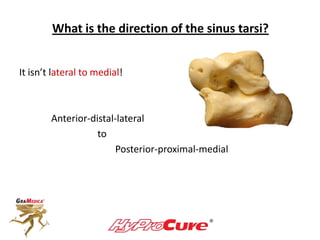
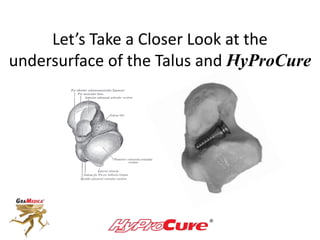
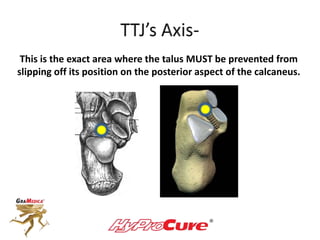
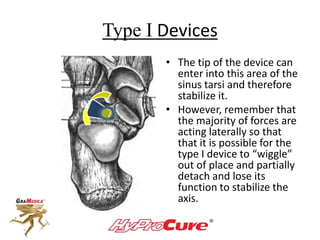
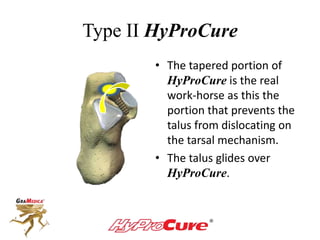
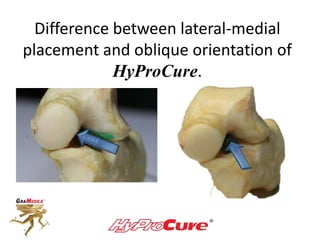
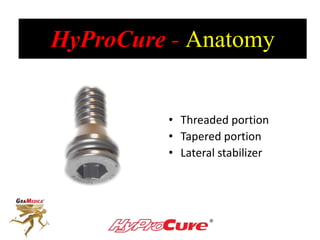
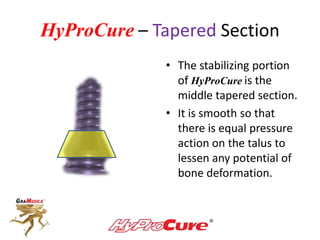
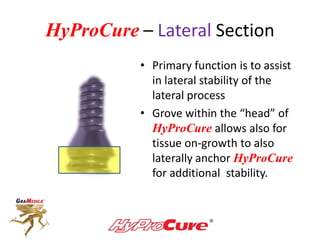
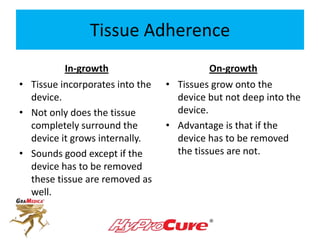
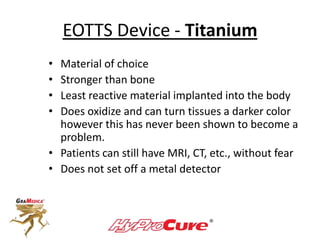
This document discusses different types of sinus tarsi devices. Type I devices are laterally anchored in the outer portion of the sinus tarsi, while Type II devices are medially anchored deeper in the sinus tarsi canalis portion. The HyProCure device is classified as a Type II device that is inserted at an oblique angle from anterior-lateral to posterior-medial, stabilizing the talus more effectively than Type I devices. Its unique tapered design and titanium composition provide strong stabilization of the talotarsal joint without risk of displacement.