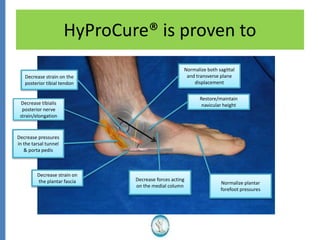
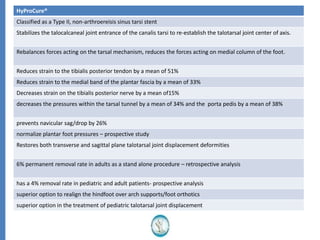
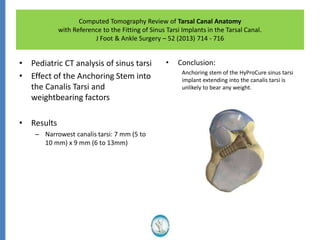
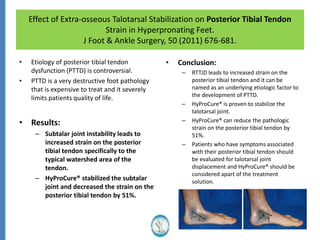
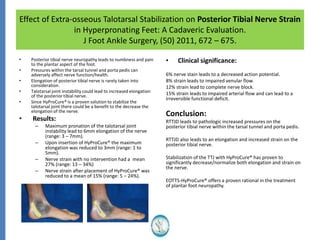
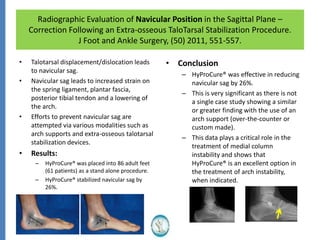
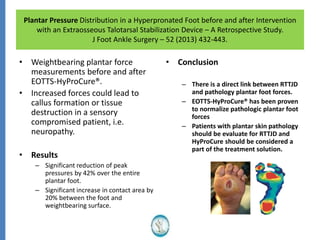
The document reviews the safety and efficacy of the Hyprocure sinus tarsi stent, which is FDA cleared and CE marked, showing significant benefits for patients with foot pathologies by stabilizing the talocalcaneal joint and reducing strain on related structures. With a low removal rate of 4-6%, Hyprocure has demonstrated superior outcomes when compared to traditional arch supports and arthroereisis devices, making it a preferred option for treating conditions such as talotarsal joint displacement. The extensive clinical evidence supports its efficacy in reducing pain and normalizing foot mechanics in both pediatric and adult populations.