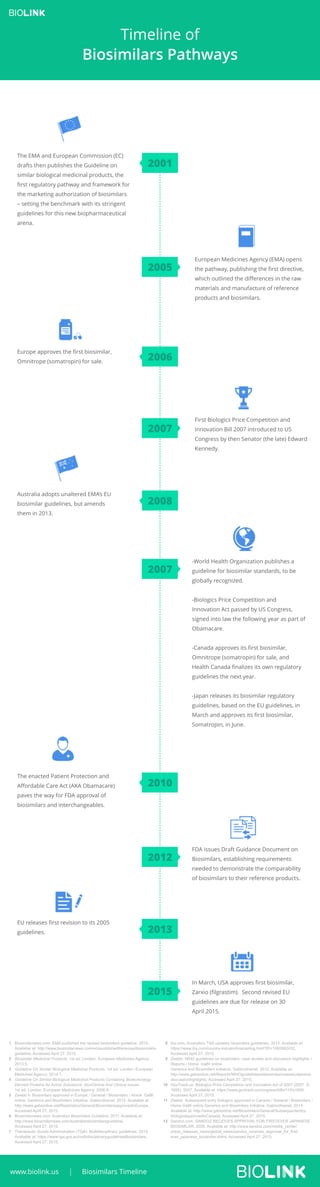
The document outlines the timeline of biosimilars pathways, detailing significant regulatory developments from the European Medicines Agency (EMA) and global entities. Key milestones include the publication of guidelines for biosimilars by the EMA, the approval of various biosimilars in Europe, the U.S., Canada, and Japan, and the enactment of the Biologics Price Competition and Innovation Act. It highlights the increasing regulatory frameworks designed to ensure the quality and efficacy of biosimilars since the mid-2000s.