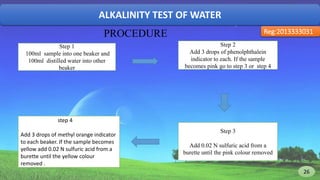
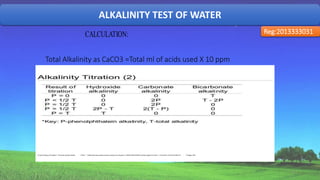
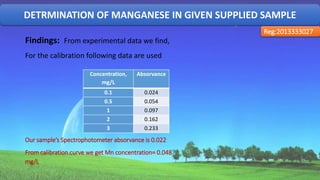
The document discusses the analysis of various water quality parameters from a sample collected from the 2nd ladies hall of SUST. It provides results for pH, carbon dioxide, turbidity, alkalinity, iron, and total solids, dissolved solids, and suspended solids. The results are within drinking water standards except for the pH, which indicates the water sample is acidic and further testing of other parameters is required before the water can be deemed safe for drinking.





![DETERMINATION OF pH OF WATER
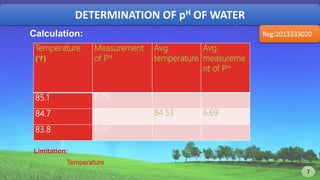
Reg:2013333020PH=-log10[H+]
Measure- acid or alkaline condition of water.
PH is important for,
Water supply.
Sewage treatment.
Chemical process plant.
Biological treatment.
Drinking water.
Use-digital PH meter.
-Electrometric method.
6 6](https://image.slidesharecdn.com/watersupplyandsewerageengineering-180202202635/85/Water-supply-and-sewerage-engineering-6-320.jpg)







































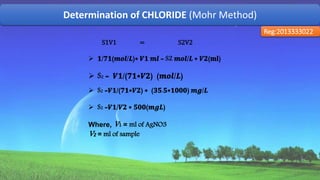
![Determination of CHLORIDE (Mohr Method)
Reg:2013333022
Volume of
sample, V2
(ml)
Con. Of
𝐻2 𝑆𝑂4, S1
(mol)
Volume of
𝐻2 𝑆𝑂4, V1
(ml)
Con. Of
sample, S2=
( V1 – 0.2 )*
10 (mol)
50 1/71 1.1 9
Chloride content, mg/l = [(ml of AgNO3 used – 0.2*) x
500] / [ml of sample]
55](https://image.slidesharecdn.com/watersupplyandsewerageengineering-180202202635/85/Water-supply-and-sewerage-engineering-55-320.jpg)

