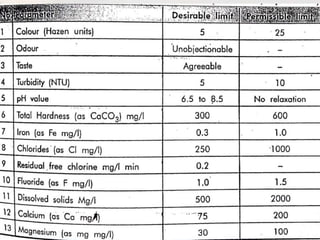
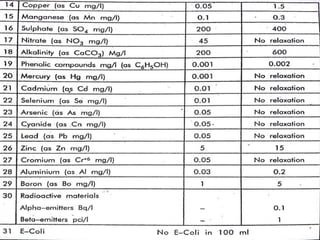
This document discusses various aspects of water pollution including its definition, sources, parameters used to measure it, and methods for controlling it. Water pollution is defined as contamination that makes water harmful to living organisms. It can originate from point sources like pipes or non-point sources like agricultural runoff. Key parameters for assessing water quality include physical factors like turbidity, chemical measurements like pH levels, and biological indicators like E. coli. The document also covers topics like eutrophication, biochemical oxygen demand, and steps individuals can take to reduce pollution like proper waste disposal and water conservation.