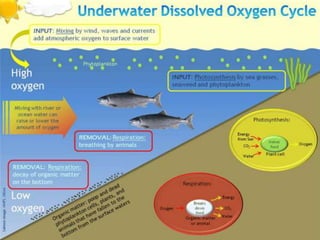
Water pollution arises from human activities and contaminates various water bodies with pollutants such as pathogens, organic waste, and heavy metals. High levels of biochemical oxygen demand (BOD) can deplete oxygen, harming aquatic life, while chemical pollutants like nitrates and lead pose risks to human health. Eutrophication occurs when nutrient runoff leads to excessive algae growth, further diminishing water quality and biodiversity.









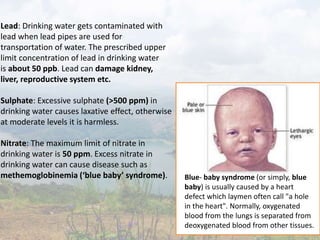
![deficiency in drinking water is harmful to
man and causes diseases such as tooth decay.
Soluble fluoride is often added to drinking
water to bring its concentration upto 1 ppm
or 1 mg dm–3. The F– ions make the enamel on
teeth much harder by converting
hydroxyapatite, [3(Ca3(PO4)2.Ca(OH)2], the
enamel on the surface of the teeth, into much
harder fluorapatite, [3(Ca3(PO4)2.CaF2].
However, F– ion concentration above 2 ppm
causes brown mottling of teeth. At the same
time, excess fluoride (over 10 ppm) causes
harmful effect to bones and teeth, as reported
from some parts of Rajasthan.](https://image.slidesharecdn.com/waterpollution-151026122516-lva1-app6891/85/Water-pollution-12-320.jpg)