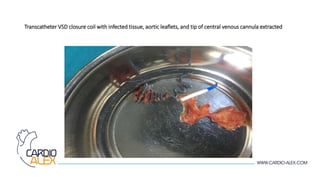
Transcatheter closure of ventricular septal defects (VSDs) is generally safe and effective but infectious endocarditis is a rare but serious complication. The case report describes a 25-year-old male who developed Pseudomonas endocarditis affecting the aortic valve and VSD closure device that was placed during childhood. Despite antibiotic treatment, the patient's condition deteriorated necessitating open heart surgery to remove the infected device and tissues, repair the VSD with a patch, and replace the damaged aortic valve. While transcatheter VSD closure has benefits over surgery, infectious endocarditis remains a serious potential complication that may require urgent intervention.