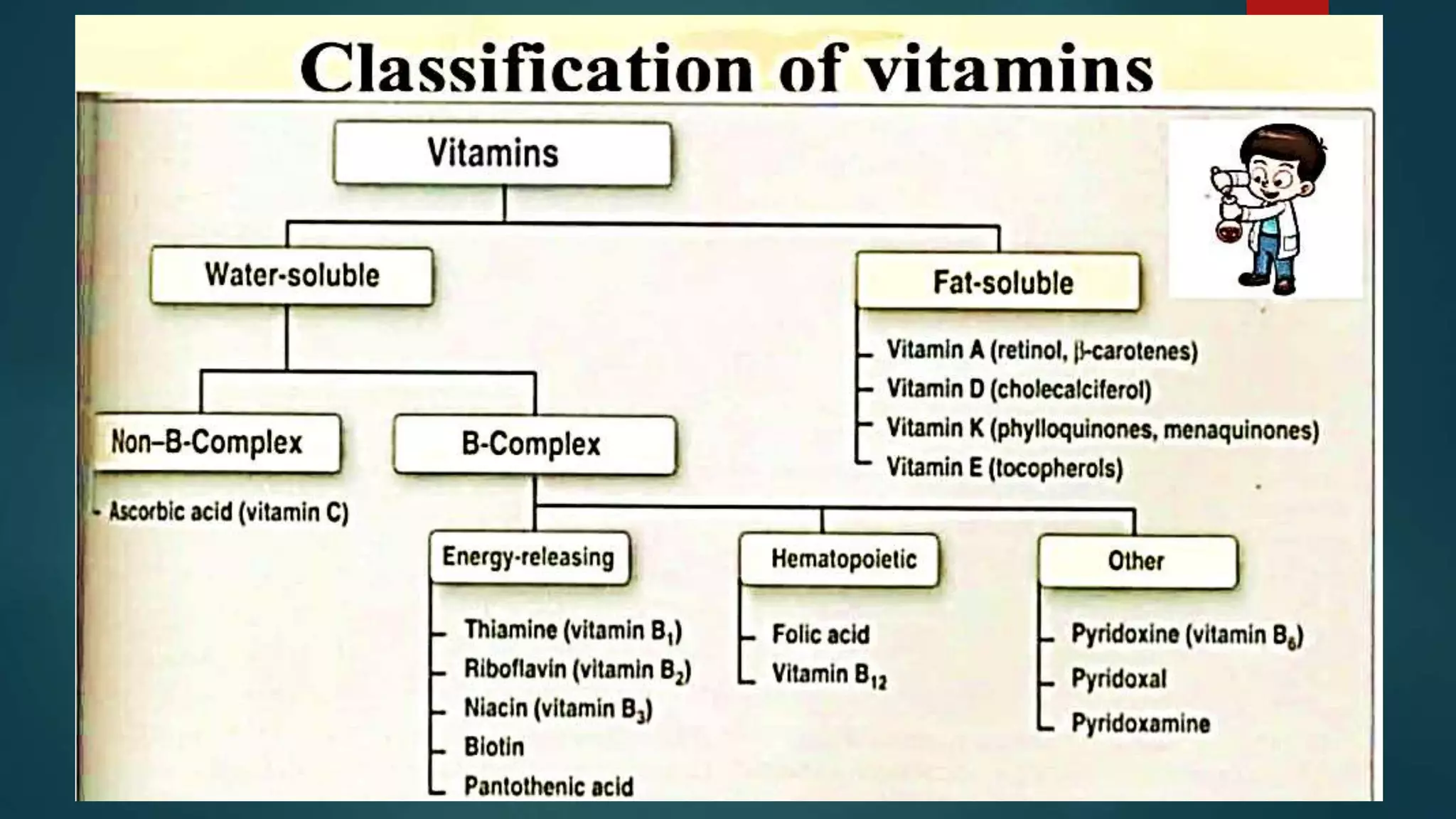
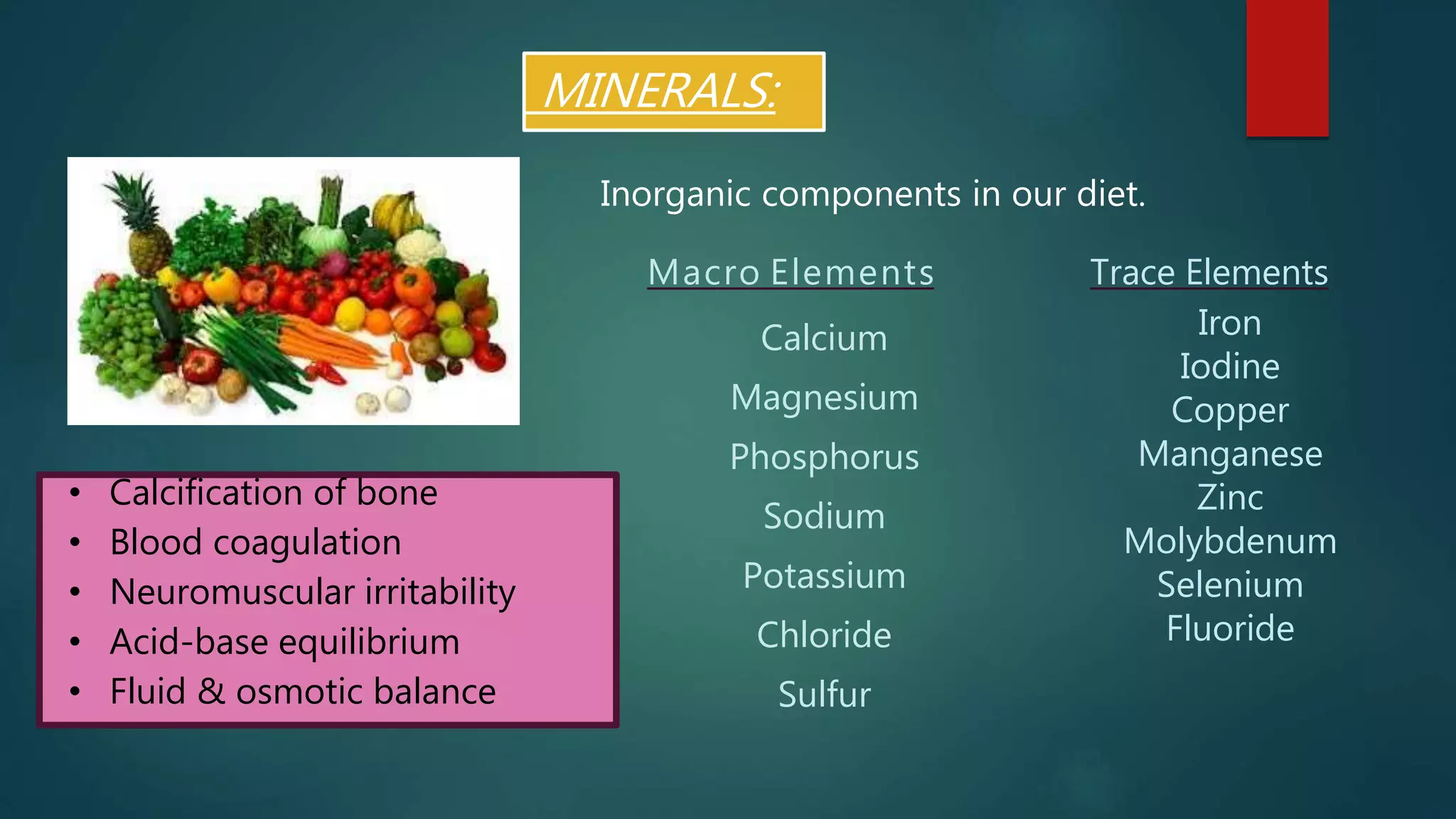
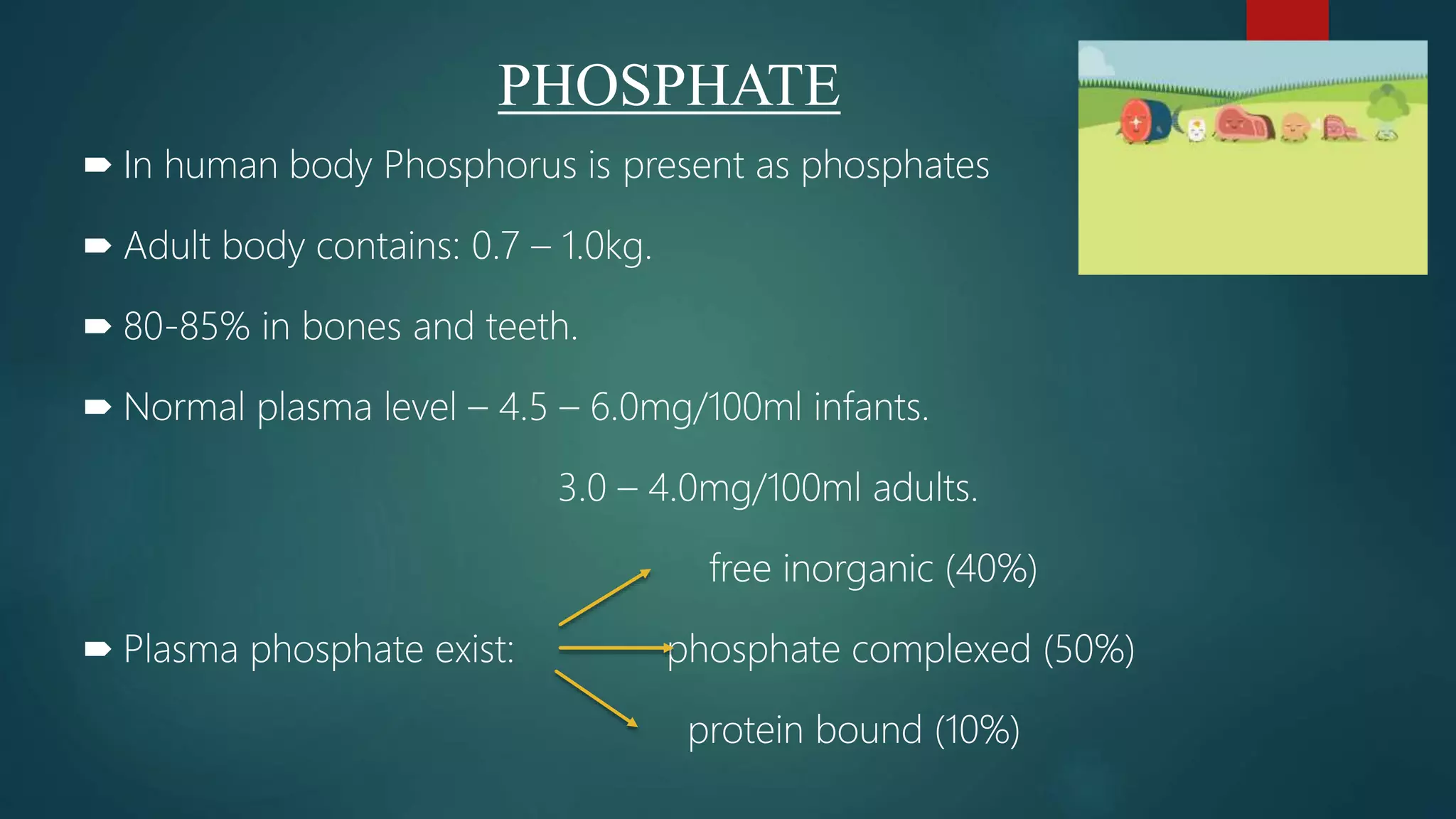
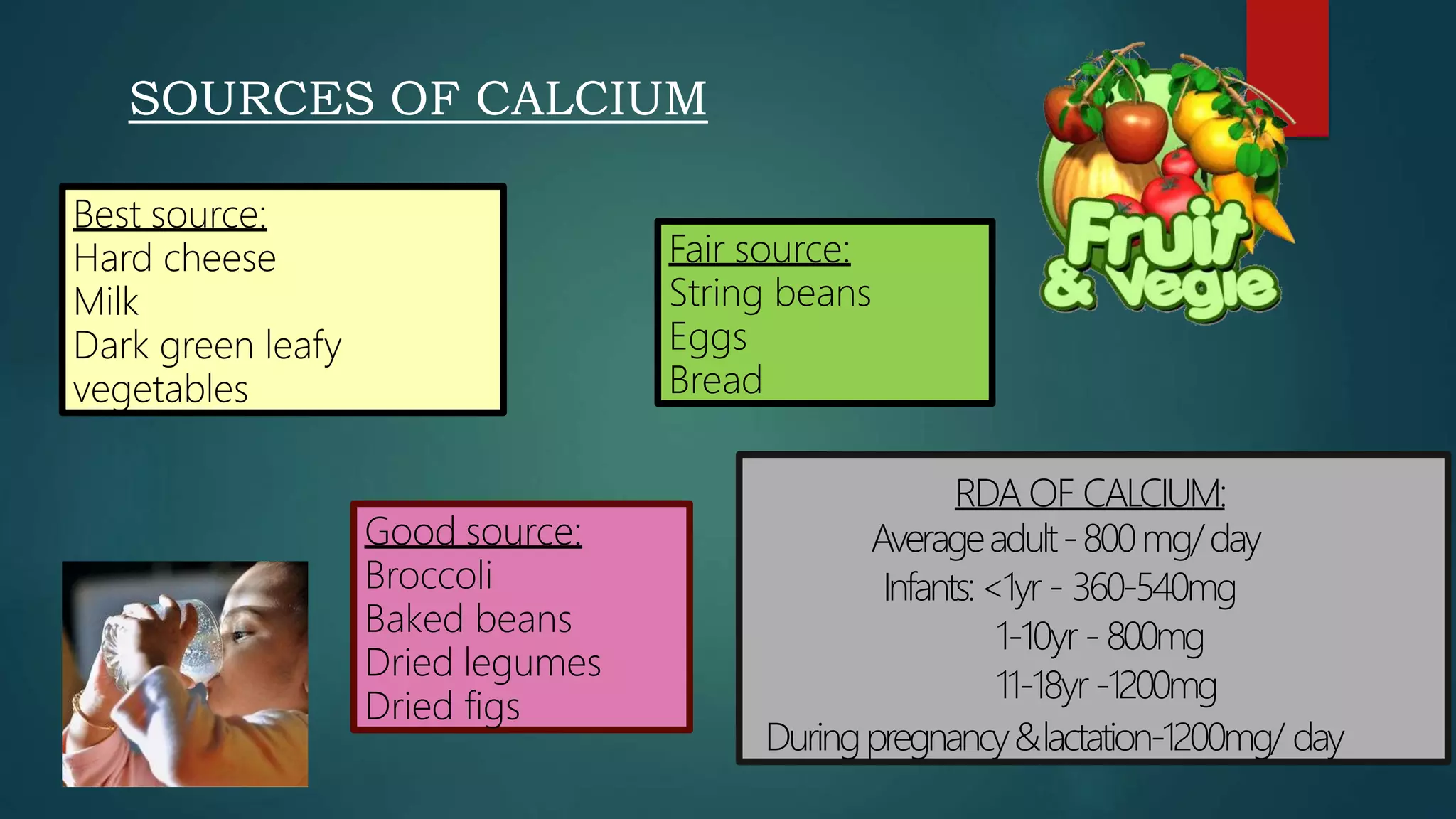
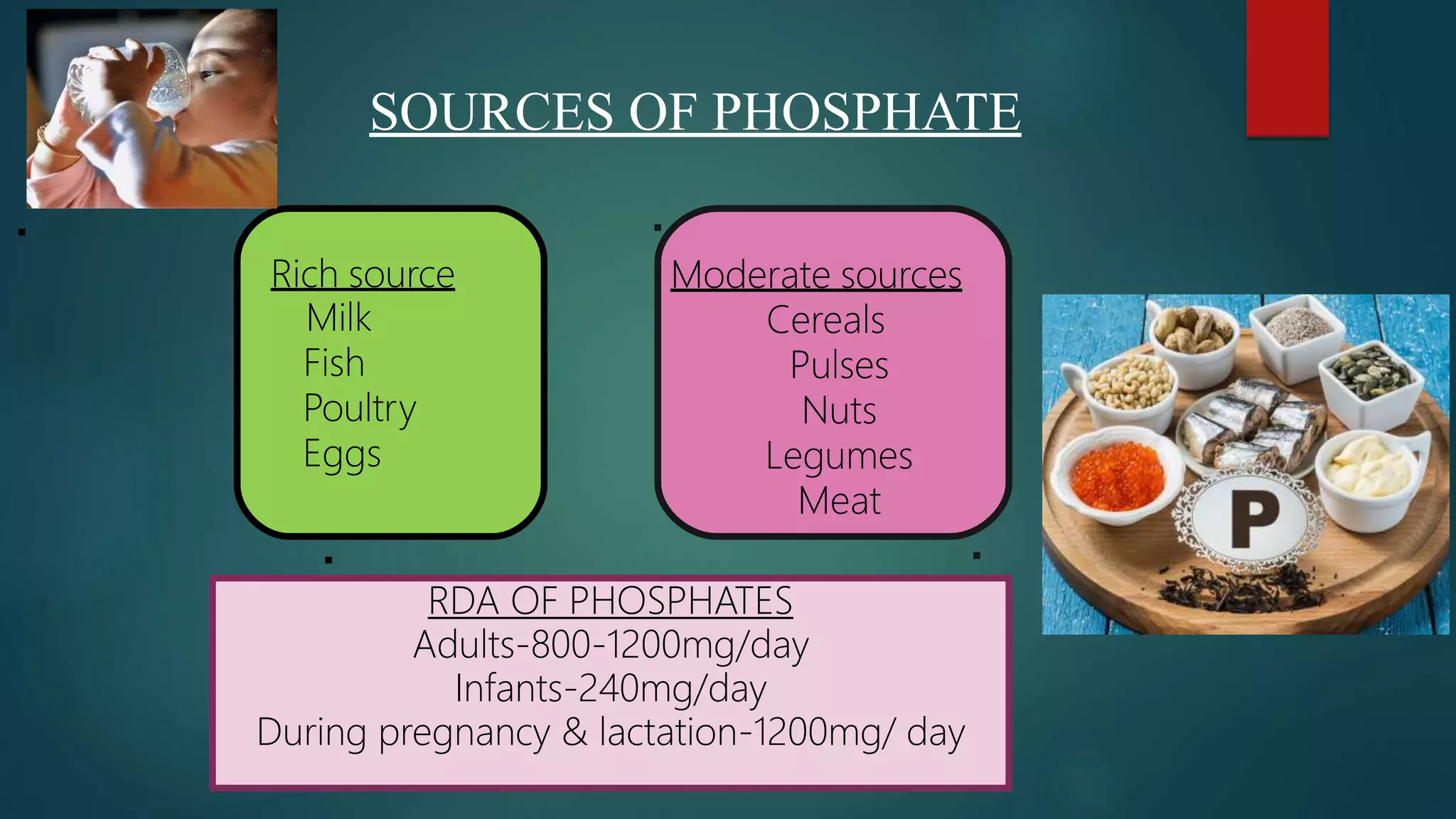
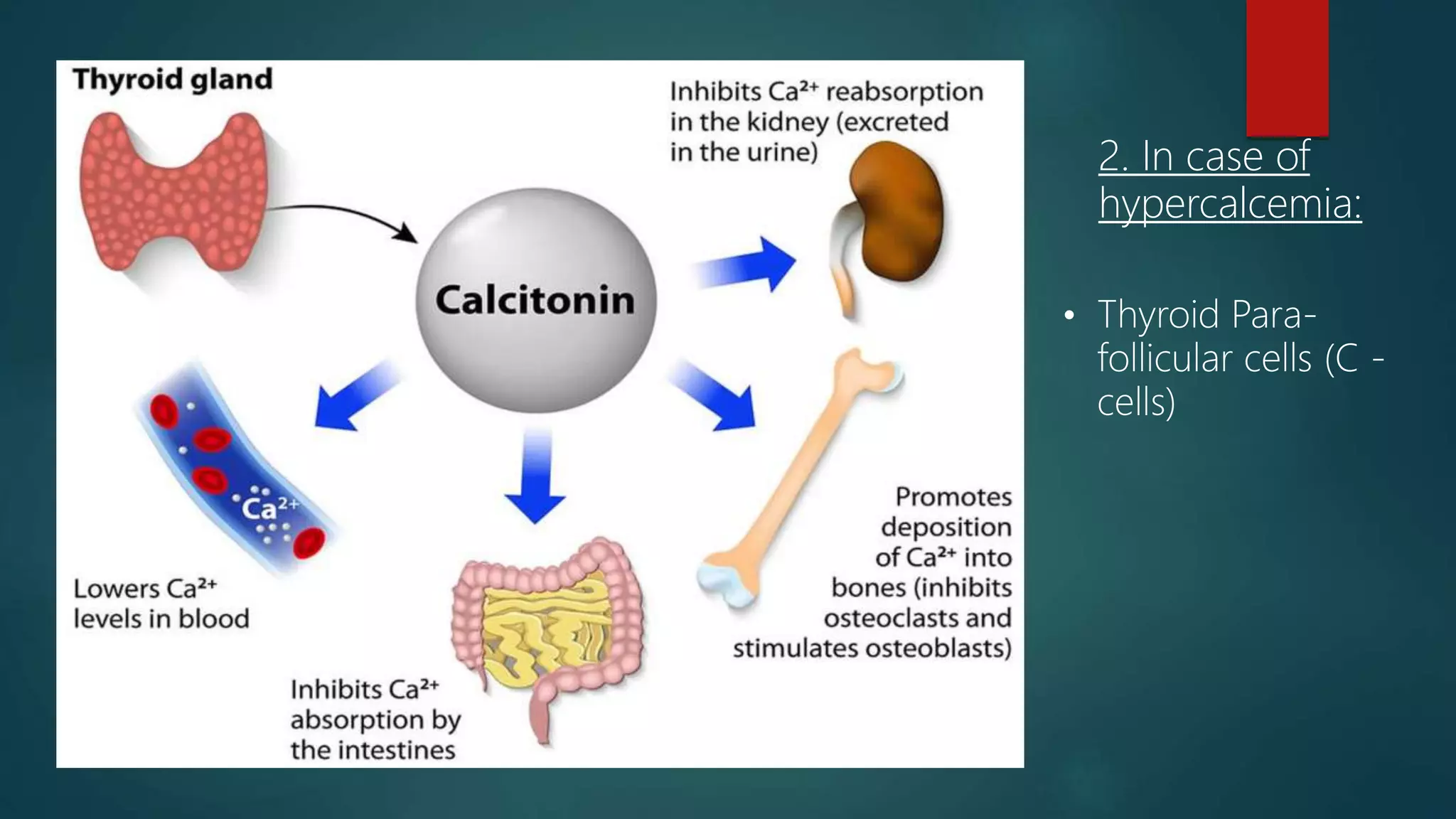
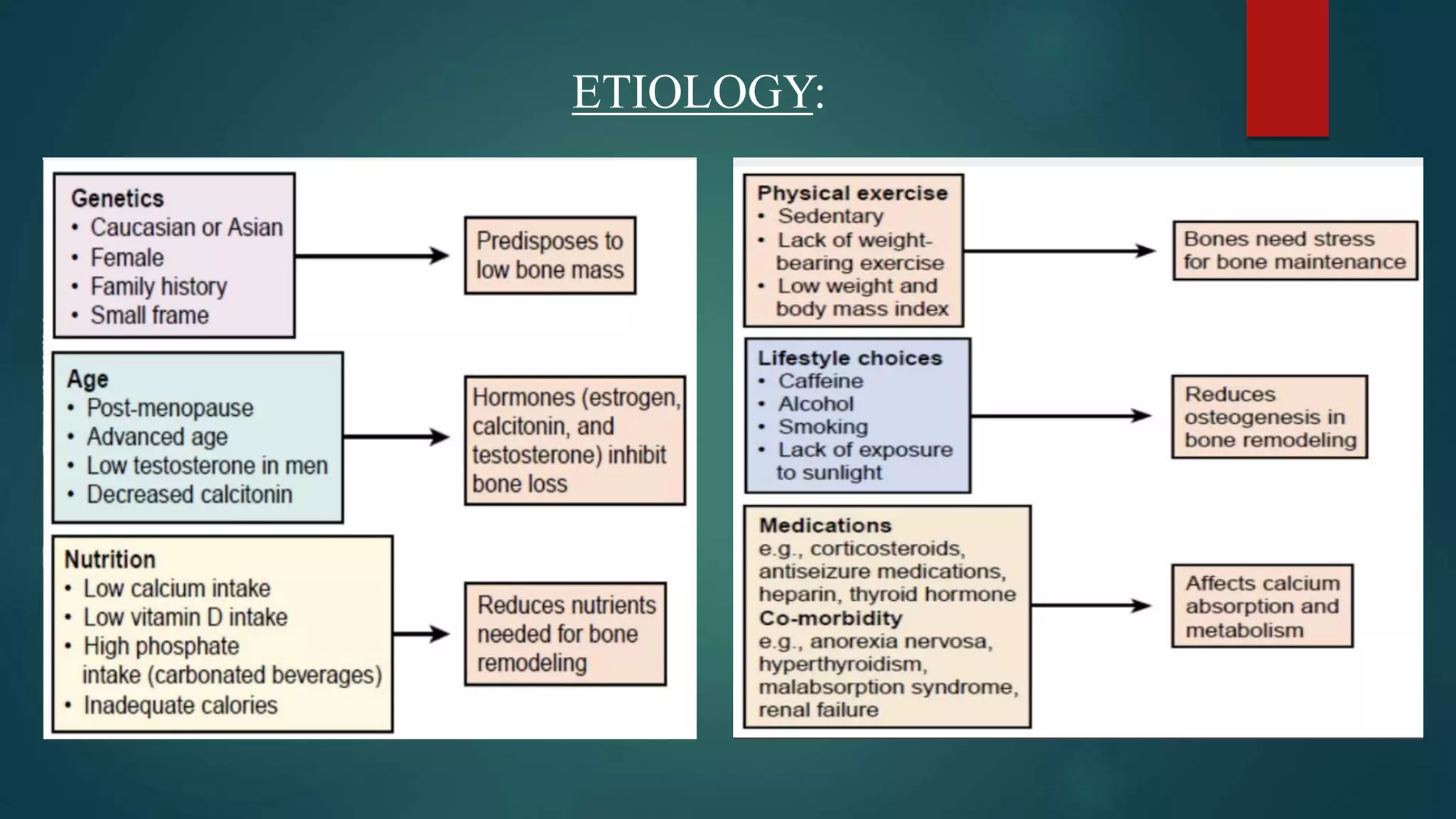
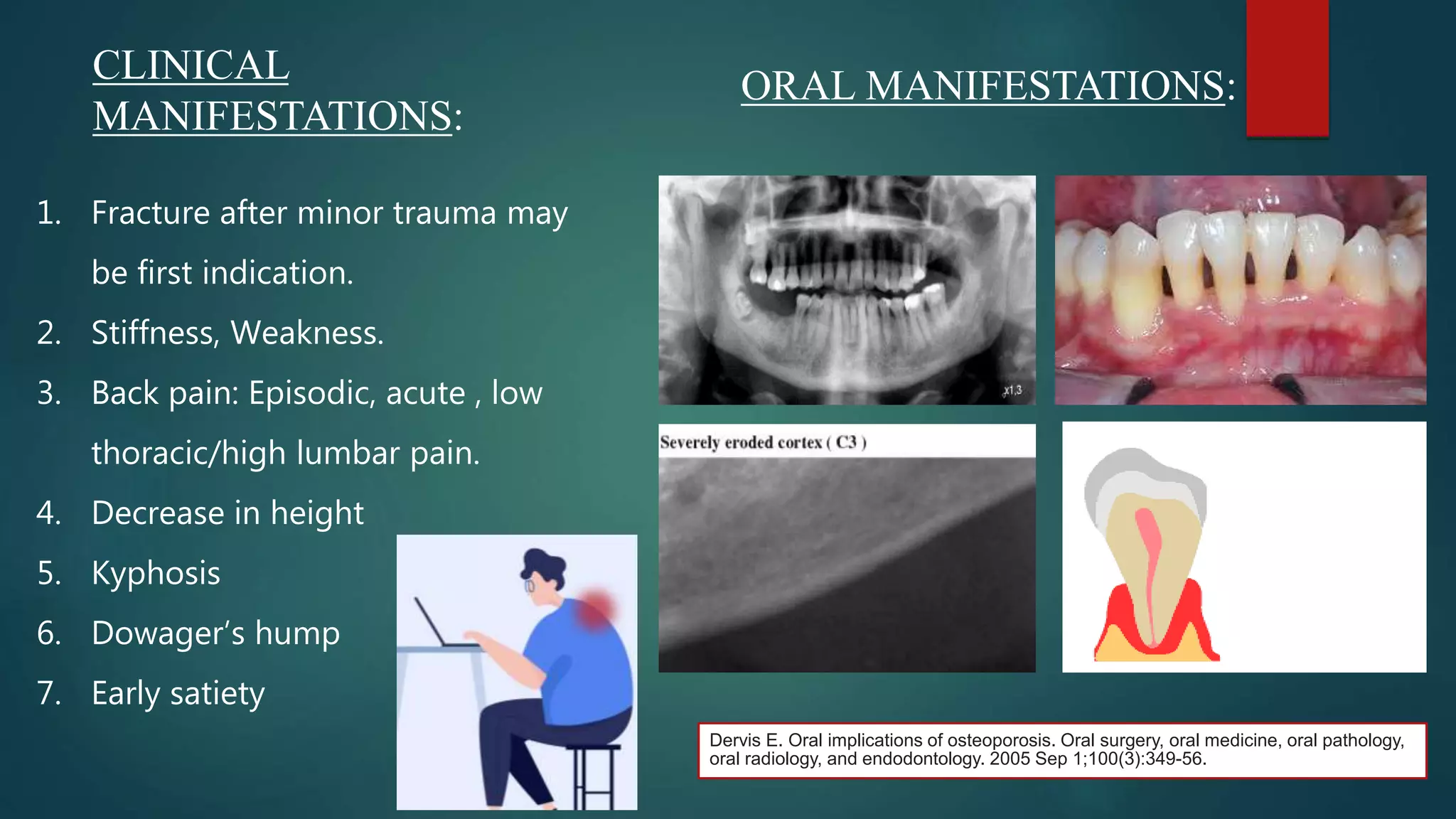
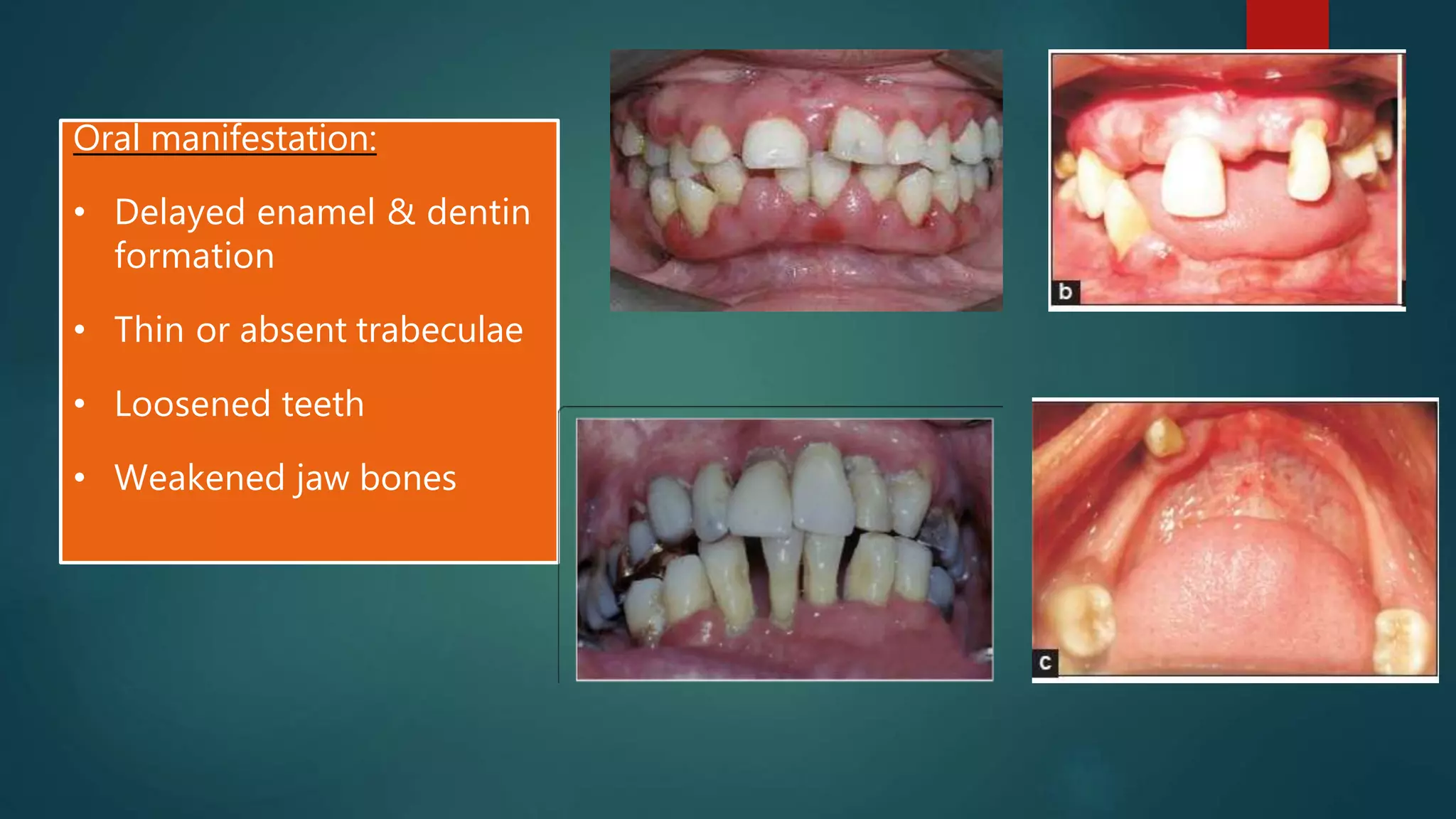
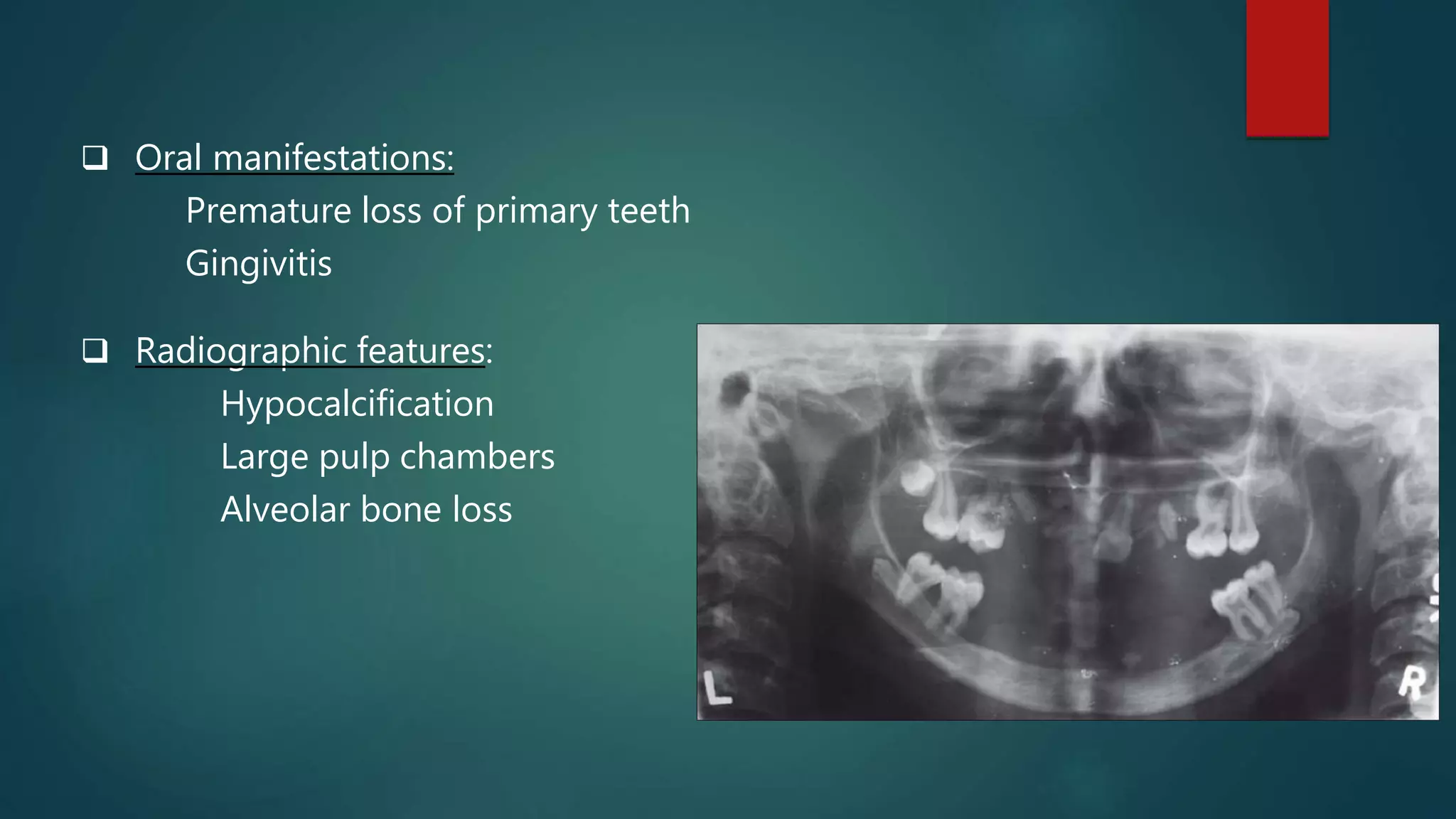
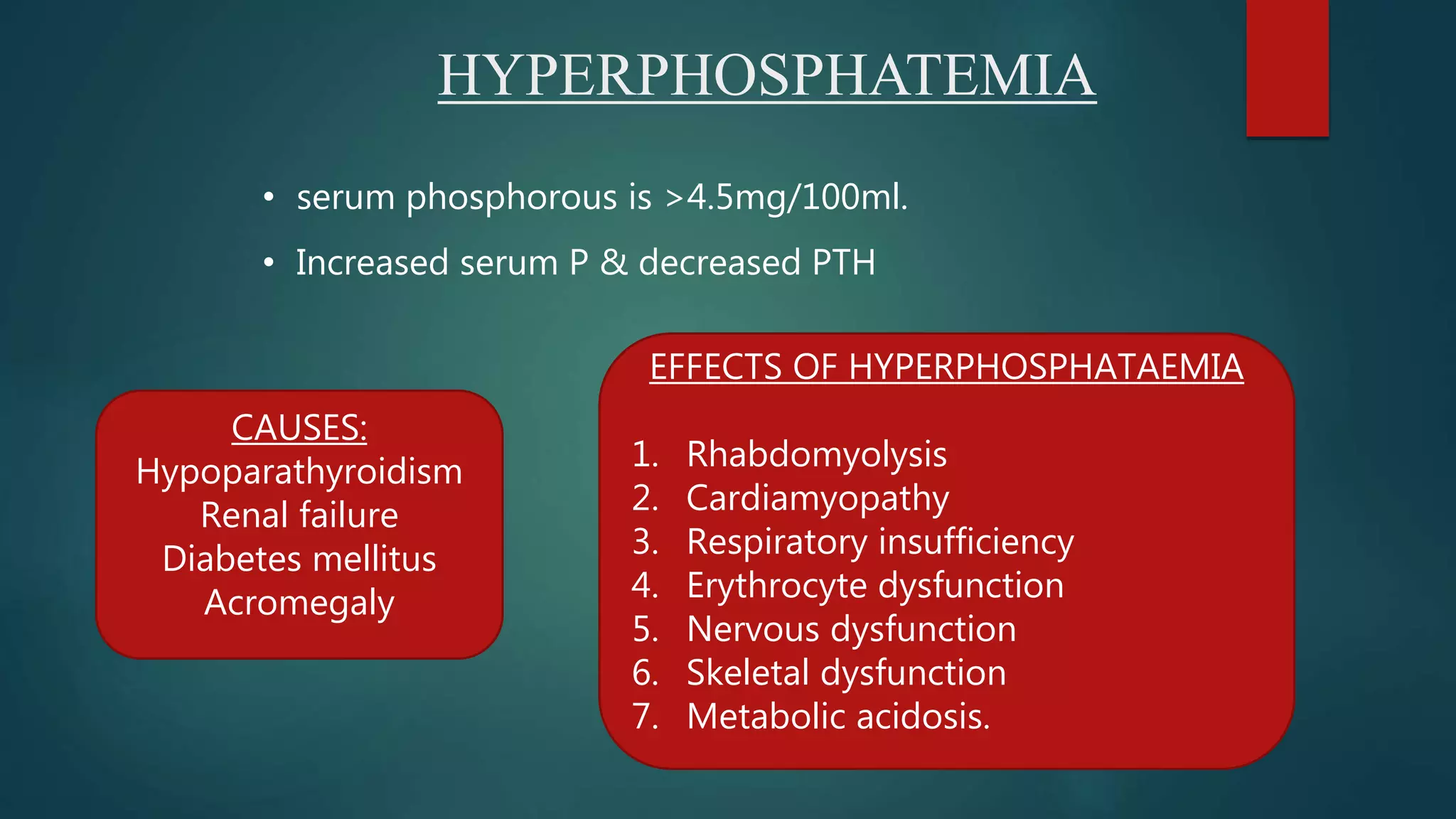
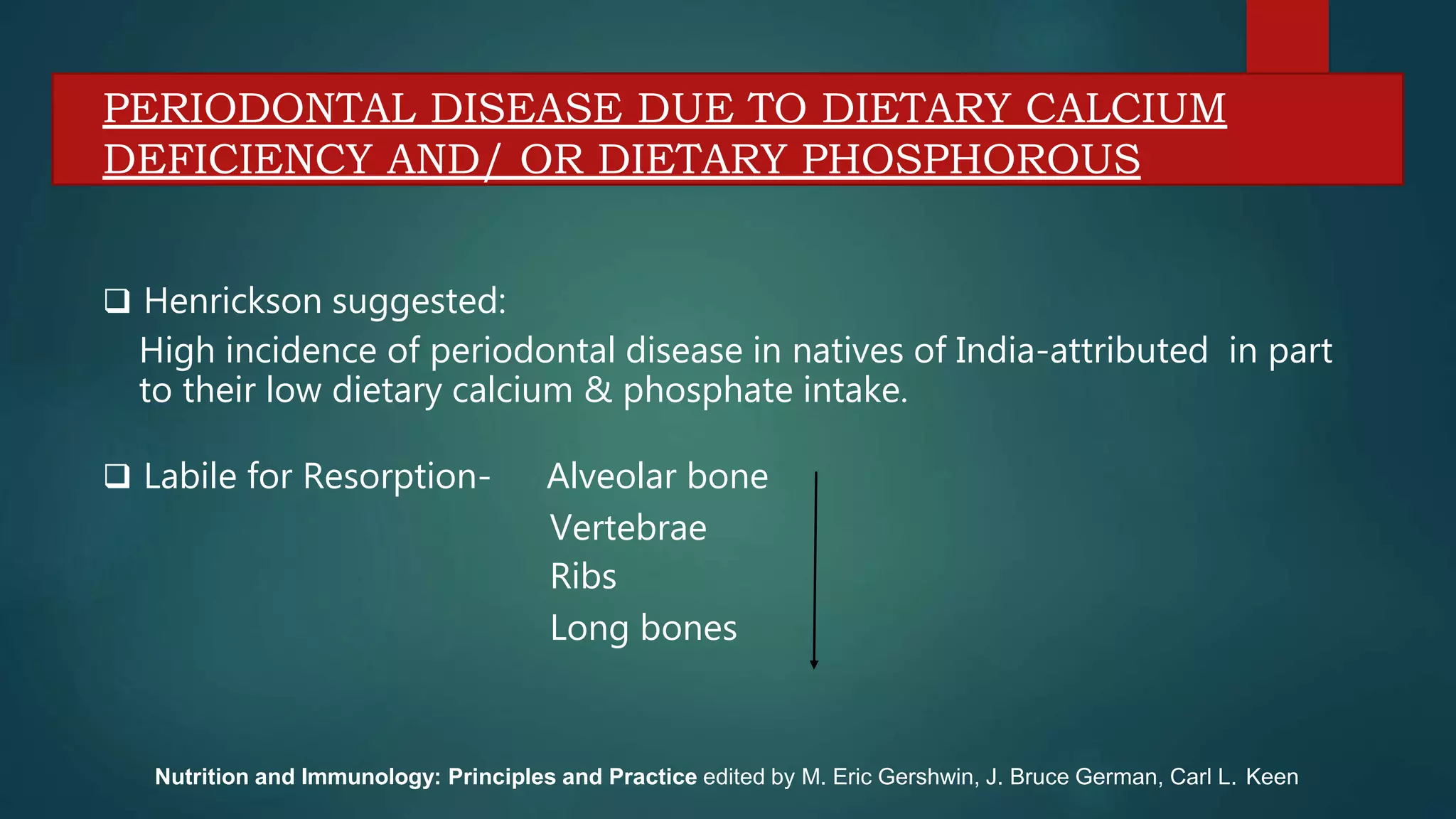
This document discusses vitamin D, calcium, and phosphate metabolism. It covers the roles and regulation of vitamin D, calcium, and phosphate in the body. Vitamin D helps regulate calcium and phosphate levels and is required for bone mineralization. Deficiencies can lead to conditions like rickets, osteomalacia, and osteoporosis. The document also discusses oral implications of these nutritional deficiencies and metabolic bone diseases. Maintaining proper levels of vitamin D, calcium, and phosphate is important for overall health and bone health.



























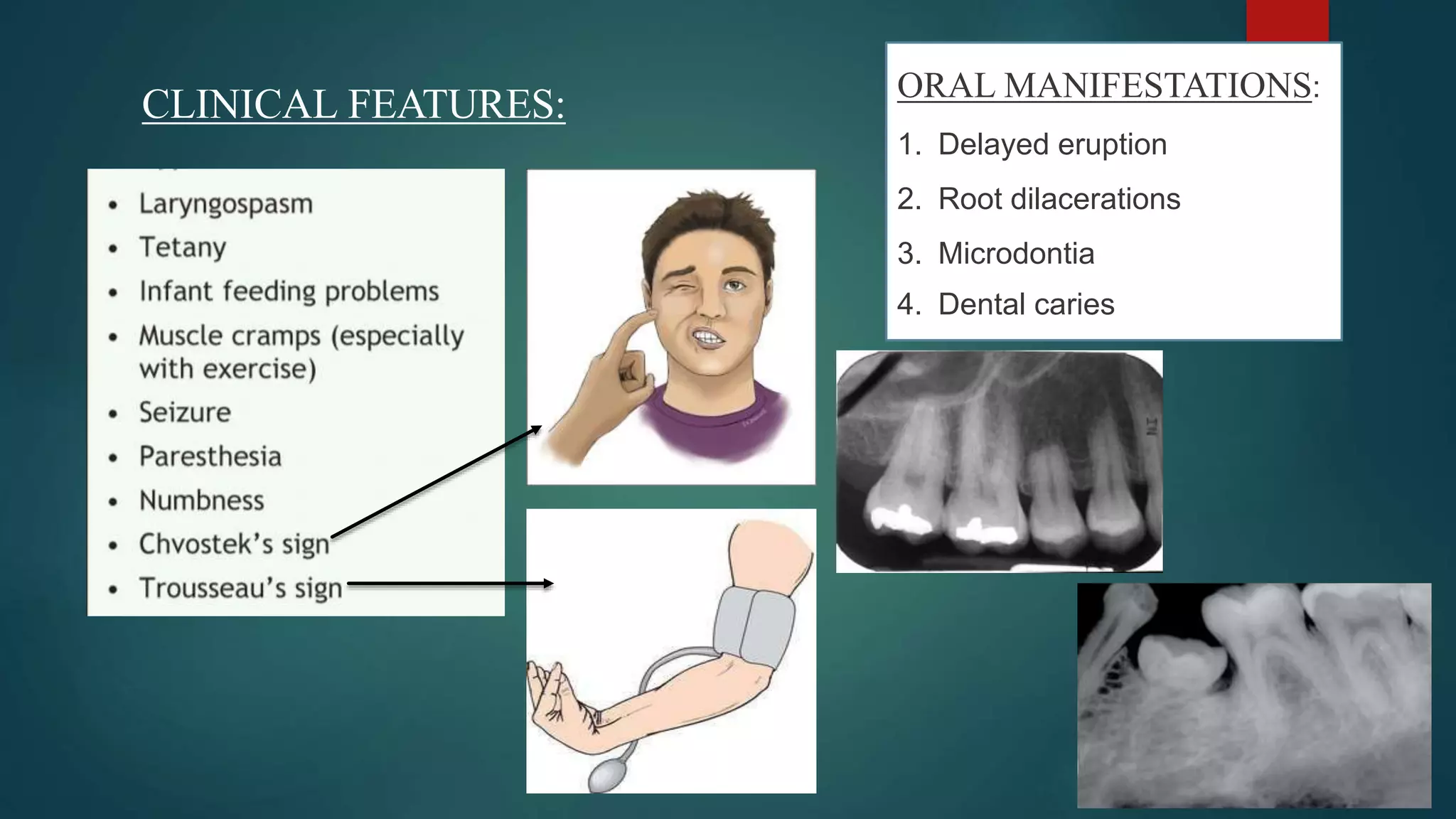
![HYPOCALCEMIA TETANY
• Plasma Ca2+ <7.5 mg/100ml.
• For each gram decrease of albumin from
normal (i.e., 4.0 mg/100ml), [Ca2+] decreases
by 0.8 mg/100ml.](https://image.slidesharecdn.com/vitamind-210322192724/75/VITAMIN-D-CALCIUM-PHOSPHATE-METABOLISM-37-2048.jpg)







![REFERENCES
1. Guyton’s Textbook of Medical Physiology; 8th edition.
2. Biochemistry by Dr. U Satyanarayana 3rd edition.
3. Textbook of biochemistry with biochemical significance by Prem Prakash
Gupta.
4. Ferguson, John H. (1936). THE BLOOD CALCIUM AND THE CALCIUM
FACTOR IN BLOOD COAGULATION. Physiological Reviews, 16(4), 640–
670.
5. Bolat M, Chiriac MI, Trandafir L, Ciubara A, Diaconescu S. Oral
manifestations of nuritional diseases in children. Romanian Journal of Oral
Rehabilitation. 2016 Apr 1;8(2):56-60.
6. Mizumoto T. Effects of the calcium ion on the wound healing process.
[Hokkaido igaku zasshi] The Hokkaido journal of medical science. 1987
Mar;62(2):332.](https://image.slidesharecdn.com/vitamind-210322192724/75/VITAMIN-D-CALCIUM-PHOSPHATE-METABOLISM-50-2048.jpg)
