This study evaluated the long-term efficacy, safety, and stability of posterior chamber phakic intraocular lens (ICL) implantation for correction of high ametropia. The study retrospectively reviewed 90 eyes of 53 patients with high myopia, hyperopia, or astigmatism who underwent ICL implantation with a mean follow-up of 5 years. Results showed that ICL implantation effectively corrected refractive error and achieved good visual acuity outcomes. Post-implantation, endothelial cell density decreased stable at 0.69% per year and few eyes developed lens opacity. Overall patient satisfaction was high, demonstrating that ICL implantation is an effective option for correcting high ametropia with a favorable long-term safety

![Modele +
JFO-489; No. of Pages 10 ARTICLE IN PRESS
2 M. Le Loir, B. Cochener
Conclusion. — Les résultats sont en faveur de l’efficacité, la stabilité et la sécurité de l’implant
phaque ICL V4 dans le traitement des amétropies fortes. Le suivi au long cours n’a pas mis en
évidence d’augmentation significative d’incidence de cataracte dans les yeux opérés.
© 2012 Publi´ par Elsevier Masson SAS.
e
KEYWORDS Summary
Phakic intraocular Purpose. — To assess efficacy, stability and safety of posterior chamber phakic intraocular lens
lens implantation; implantation with STAAR Visian ICL for correction of high ametropia, with a mean follow-up of
Phakic IOL; 5 years (3.5—10 years).
ICL; Patients and methods. — Ninety eyes of 53 highly ametropic patients (45 myopia, ten hyperopia
Long-term follow-up and 35 with mixed astigmatism) were included in a retrospective single-surgeon study, using
primarily the V4 ICL model (87 eyes). We studied pre- and postoperative refractive efficacy,
endothelial cell density, crystalline lens opacification and intraocular clearances within the
various compartments of the eye.
Results. — Mean uncorrected visual acuity was 0.77 at the 12th postoperative month; 17 of 90
eyes required adjunctive photoablation for residual astigmatism. Forty-eight percent of eyes
gained at least one line of best corrected visual acuity. After implantation, the decrease in
endothelial cell density remained stable at 0.69%/year, and 91% of eyes showed no opacifi-
cation of the crystalline lens. Mean endothelium-ICL and ICL-crystalline lens distances were
2.41 mm and 0.52 mm respectively. Overall patient satisfaction achieved was 96% at 36 months
postoperatively.
Discussion and conclusion. — These results demonstrate efficacy, stability and safety of the
ICL V4 phakic IOL for the correction of high ametropia. Long-term follow-up did not show a
significant increase in cataract formation in implanted eyes.
© 2012 Published by Elsevier Masson SAS.
Introduction chirurgie incisionnelle, l’implantation intraoculaire torique
et la photoablation secondaire. Enfin, la grossesse est une
L’implantation phaque représente l’option chirurgicale contre-indication transitoire [1—3].
réfractive de choix pour la correction des amétropies fortes L’implantation phaque de chambre antérieure est deve-
(myopie supérieure à neuf dioptries, hypermétropie et astig- nue impopulaire en raison de complications tardives
matisme supérieurs à quatre dioptries). Elle reste une obtenues avec les implants à appui angulaire et à un moindre
alternative en cas d’intolérance aux lentilles de contact degré avec les implants à fixation irienne [4—8]. La plus
ou de contre-indication au LASIK (cornée fine ou oblate, redoutée est l’œdème cornéen par perte cellulaire endo-
opacités cornéennes, enophtalmie. . .). Au-delà des limites théliale, lié au contact mécanique des anses en appui sur
de la photoablation, elle respecte la cornée (et sa prola- l’endothélium, aux microtraumatismes mettant en contact
ticité), autorise une meilleure qualité de vision, offre une endothélium et implant au sein d’une chambre antérieure
réversibilité réfractive et anatomique, et enfin permet un trop étroite, ou à une mauvaise biotolérance du matériau
éventuel traitement photoablatif complémentaire (Bioptic). de l’implant. Citons également l’ovalisation pupillaire et la
Cette technique est le plus souvent réalisée de facon bila-
¸ cataracte précoce. Ces complications ont conduit au retrait
térale chez des patients âgés de 20 à 40 ans. du marché de la quasi-totalité des implants phaques de
Les contre-indications actuelles sont les suivantes : une chambre antérieure à appuis angulaires (à l’exception de
infection chronique des annexes oculaires, un antécédent de l’Acrysof phaque [Alcon® ]) et à la nécessité d’un suivi rigou-
chirurgie oculaire, de pathologie inflammatoire cornéenne reux à long terme [9,10].
et intraoculaire, de pseudoexfoliation ou de dispersion pig- L’implantation phaque de chambre postérieure peut être
mentaire, une insuffisance endothéliale (< 2000 cell/mm2 ), réalisée grâce à deux modèles d’implants. Le plus uti-
une hypertonie oculaire ou un glaucome, une opacification lisé, l’ICL (Implantable Collamer Lens, distribué par Staar
cristallinienne même débutante, un antécédent de décolle- Surgical® ) est constitué d’un matériel flexible et hydrophile,
ment rétinien ou de pathologie maculaire (à exclure par OCT le Collamer, dont l’indice de réfraction est de 1,45. Sa lar-
et/ou angiographie fluorescéinique), tout patient porteur geur est de 7,0 mm et la détermination de sa longueur (qui
d’une pathologie générale telle que le diabète sucré, une varie de 11,5 à 13 mm) repose en partie sur la distance
maladie auto-immune ou une pathologie systémique sévère « blanc à blanc » de limbe à limbe horizontal, approxima-
ou soumis à un traitement immunosuppresseur. De plus, tion du diamètre du sulcus ciliaire. L’optique, plan-concave,
comme dans toute chirurgie réfractive, en cas d’amblyopie présente un diamètre compris entre 4,5 et 5,5 mm selon la
minime et modérée, il faut prévenir le patient des limites puissance dioptrique de l’implant. Cet implant se positionne
de récupération. De même tout astigmatisme supérieur à en chambre postérieure, ses haptiques étant positionnées
1,5 dioptries ne constitue pas une contre-indication abso- dans le sulcus ciliaire (Fig. 1—3). Le PRL (Phakic Refrac-
lue mais il faudra évoquer la possibilité de choix parmi la tive Lens, distribué par Zeiss® ) fait de silicone, souple et
Pour citer cet article : Le Loir M, Cochener B. Résultats à long terme de l’implantation phaque de chambre postérieure
pour la correction des amétropies fortes. J Fr Ophtalmol (2012), doi:10.1016/j.jfo.2011.06.006](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-2-320.jpg)
![Modele +
JFO-489; No. of Pages 10 ARTICLE IN PRESS
Implantation phaque de chambre postérieure pour correction des amétropies fortes 3
Figure 3. Implant ICL en position précristallinienne (flèche)
lors d’un examen biomicroscopique en mydriase thérapeutique.
L’implant apparaît à distance de l’endothélium cornéen, et à dis-
tance de la cristalloïde antérieure (vault).
Depuis 1998, de nombreuses études ont démontré
l’efficacité et la prédictibilité réfractive de l’implantation
phaque de chambre postérieure [13—20] avec des résul-
Figure 1. Implant ICL sphérique (STAAR Surgical) souple, consti- tats comparables à ceux obtenus avec les implants phaques
tué de Collamer, de largeur 7 mm et de longueur comprise entre de chambre antérieure [21—25]. En revanche, la durée de
11,5 et 13 mm, avec une optique plan-concave de 4,5 à 5,5 mm de suivi souvent inférieure à trois années [13—20], ne permet-
diamètre selon la puissance. Les haptiques sont au contact du sulcus
tait pas de valider la sécurité de la technique vis-à-vis de
ciliaire.
complications à long terme : opacification cristallinienne,
perte cellulaire endothéliale, syndrome de dispersion pig-
élastique, repose théoriquement sur les fibres zonulaires et
mentaire, glaucome pigmentaire et blocage pupillaire.
se positionne librement en chambre postérieure.
Récemment, Kamiya et al. [26] ont conclu à l’efficacité
L’implantation précristallinienne est à ce jour restée
réfractive et la sécurité de l’implantation ICL avec un recul
de diffusion timide en France du fait de sa réputation
prolongé à 4 ans pour la correction des myopies comprises
d’inducteur de cataracte précoce de type sous-capsulaire
entre −4 et −15 dioptries. Pesando et al. [27] ont effec-
antérieur, notamment pour les premières générations d’ICL.
tué une étude avec dix ans de suivi mais uniquement sur
Mais la validation par la Food and Drug Administration
des patients hypermétropes. Notre travail est original à plu-
d’implants ICL V4 (quatrième génération) au dessin optimisé
sieurs titres. Avec un recul moyen proche de cinq ans, il
et le recul des implants de chambre antérieure expliquent
traite de l’implantation ICL pour corriger non seulement
sa diffusion exponentielle dans le monde [11—13].
les myopies, mais aussi les hypermétropies et astigmatismes
modérés à sévères, en s’affranchissant du biais « opérateur-
dépendant ».
Notre étude réalisée à l’aide de l’ICL V4 propose
d’évaluer l’efficacité réfractive et abérrométrique mais
également les sécurités anatomiques (endothéliale, cris-
tallinienne, angulaire irido-cornéenne) et pressionnelle
intraoculaire grâce à un suivi régulier et prolongé à dix ans.
Patients et méthodes
Nous avons réalisé une étude monocentrique rétrospec-
tive sur la période août 1998—novembre 2008, incluant
90 yeux de 53 patients forts amétropes (myopie comprise
entre − 6 et − 23 D, hypermétropie comprise entre + 4,5 et
+ 10 D ou porteurs d’un astigmatisme combiné compris entre
1,75 à 3,25 D), âgés de 18 à 44 ans, intolérants aux len-
Figure 2. Implant ICL torique (STAAR Surgical) positionné en tilles de contact et ne présentant pas de contre-indication
chambre postérieure ; les repères axiaux (flèches) diamétralement à l’implantation phaque. Tous les patients ont été opé-
opposés, et visualisables en mydriase extrême, sont alignés avec rés par le même chirurgien, à l’aide de l’implant phaque
l’axe de l’astigmatisme préopératoire. de chambre postérieure ICL STAAR® (3 V3 et 87 V4). La
Pour citer cet article : Le Loir M, Cochener B. Résultats à long terme de l’implantation phaque de chambre postérieure
pour la correction des amétropies fortes. J Fr Ophtalmol (2012), doi:10.1016/j.jfo.2011.06.006](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-3-320.jpg)



![Modele +
JFO-489; No. of Pages 10 ARTICLE IN PRESS
Implantation phaque de chambre postérieure pour correction des amétropies fortes 7
probablement liés à la rémanence de solution viscoélastique
au niveau du trabéculum.
Qualité de vision
Enfin, la qualité de vision subjective relevée au 48e mois
postopératoire souligne que seuls trois patients se plaignent
d’éblouissement, deux patients présentent des halos noc-
turnes, et deux autres patients décrivent un inconfort visuel.
Au sixième mois postopératoire, à la question : « Referiez-
vous la chirurgie ? » 96 % des patients répondent « oui ». La
qualité de vision objectivée par l’aberrométrie, retrouve
un taux d’aberrations d’ordre élevé notablement bas (RMS
hoa moyen égal à 0,25 [± 0,12]) pour un RMS total moyen
égal à 0,89 ± 0,32 et un Blurry effect hoa moyen égal à
0,21 ± 0,13 pour un Blurry effect total moyen égal à 0,63
Figure 11. Évolution de la distance séparant les centres de la face (± 0,28) au 36e mois postopératoire, mais l’échantillon étu-
postérieure de l’ICL et de la cristalloïde antérieure (vault) jusqu’au dié (28 yeux) est insuffisant pour être représentatif.
72e mois post-implantation ICL (STAAR Surgical).
Discussion
Les trois yeux ont bénéficié d’une bilensectomie (explan-
tation, phacoémulsification et implantation en chambre Les résultats de notre étude sont en faveur de l’efficacité
postérieure) avec un gain d’une ligne de MAVC par rapport réfractive, de la prédictibilité, de la stabilité et de la sécu-
à la situation pré-implantation phaque. rité à long terme de l’implantation ICL pour la correction
La distance moyenne séparant le cristallin de la face pos- des amétropies modérées à fortes. Depuis 1998, de nom-
térieure de l’ICL (appelée « vault ») en leur centre, a été breuses études ont démontré l’efficacité et la prédictibilité
mesurée à 0,52 ± 0,20 mm. Le « vault » ne varie significa- réfractive de l’implantation phaque de chambre postérieure
tivement ni avec le temps (p = 0,13), ni avec la dilatation [13—20] mais la durée de suivi inférieure à trois années,
pupillaire (p = 0,22) (Fig. 9—11). ne permettait pas de valider la sécurité de la technique à
long terme. Récemment, Kamiya et al. [26] ont conclu à
l’efficacité réfractive et la sécurité de l’implantation ICL
Sécurité irienne et camérulaire antérieure avec un recul prolongé à 4 ans pour la correction des myo-
pies comprises entre − 4 et − 15 dioptries. Notre étude est
L’étude de la tolérance irienne rapporte quatre cas de
originale à plusieurs titres. Avec un recul moyen proche
déformation pupillaire minime, deux cas d’hyporéactivité
de cinq ans, elle traite de l’implantation ICL pour corriger
pupillaire et huit cas de dispersion pigmentaire (dépôt
non seulement les myopies, mais aussi les hypermétropies
de pigment sur la cristalloïde antérieure). Le diamètre
et astigmatismes modérés à sévères, en s’affranchissant du
pupillaire réel préopératoire (5,73 ± 0,46 mm) n’est pas
biais « opérateur-dépendant ».
significativement modifié du premier au 48e mois suivant
En comparaison aux techniques de photoablation cor-
l’implantation (p = 0,19).
néenne, Sanders et Vukich ont démontré que l’implantation
La profondeur de chambre antérieure mesurée à l’aide de
ICL était supérieure au LASIK standard en termes d’efficacité
l’OCT de segment antérieur et du Pentacam, décroît légère-
et de sécurité pour la correction des myopies modérées à
ment (de 3,26 ± 0,24 mm en préopératoire à 3,17 ± 0,15 mm
sévères ainsi que pour la correction des myopies faibles
de facon stable jusqu’au 60e mois postopératoire) mais de
¸
[28—30]. La photoablation cornéenne, qui augmente avec
facon non significative (p = 0,14). Ajoutons que dans notre
¸
l’importance de l’amétropie à corriger est à l’origine
étude, la dilatation pupillaire n’a pas d’influence sur la pro-
d’aberrations d’ordre élevé (HOA), majorée en procédure
fondeur de chambre antérieure (p = 0,22).
LASIK standard par rapport à la procédure LASIK guidée
L’angle irido-cornéen subit une diminution d’environ
par aberromètre [31,32]. En attendant les résultats d’une
32 % après implantation (de 37 ± 6,7◦ à 25,2 ± 6,2◦ ) qui
étude randomisée comparant les deux techniques pour la
reste stable au terme du suivi. Notons qu’après dilatation,
correction des amétropies faibles à modérées, Igarashi a
l’angle irido-cornéen s’accroit significativement (p < 0,05)
démontré que l’implantation ICL induisait significativement
d’environ 25 %. (Fig. 9 et 10).
moins d’HOA et une meilleure sensibilité au contraste que
le LASIK guidé par aberrométrie pour la correction des myo-
Sécurité pressionnelle pies supérieures à − 6 dioptries [33] ; d’après Kamiya [34],
l’implantation ICL torique est supérieure au LASIK guidé par
La pression intraoculaire mesurée au tonomètre à applana- aberrométrie en termes de sécurité, efficacité, prédictibi-
tion ne semble pas influencée par l’implantation et ce, à lité et stabilité pour la correction des forts astigmatismes
long terme (13,6 ± 2,1 mmHg au 60e mois postopératoire). myopiques. L’implantation ICL induirait significativement
Nous avons rapporté trois cas d’hypertonie oculaire post- moins d’HOA du fait de la préservation du profil prolate de la
opératoire transitoires, résolus sous traitement médical et cornée [35], et une meilleure magnification rétinienne que
Pour citer cet article : Le Loir M, Cochener B. Résultats à long terme de l’implantation phaque de chambre postérieure
pour la correction des amétropies fortes. J Fr Ophtalmol (2012), doi:10.1016/j.jfo.2011.06.006](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-7-320.jpg)
![Modele +
JFO-489; No. of Pages 10 ARTICLE IN PRESS
8 M. Le Loir, B. Cochener
(± 0,24) mm, peu différent de la mesure UBM de Pitault [37]
(402 ± 194 m), et ne variant significativement ni avec le
temps ni avec la dilatation pupillaire. D’après Kamiya [40] le
« vault » diminue sensiblement avec le temps du fait du jeu
pupillaire, de l’épaississement cristallinien lié à l’âge et de
la position figée des haptiques de l’ICL ; dans la même étude,
le « vault » n’influence pas l’efficacité réfractive suggérant
qu’un positionnement strict de l’implant entre la face pos-
térieure de l’iris et le sulcus ciliaire conduit à une meilleure
prédictibilité réfractive.
Le diamètre pupillaire joue un rôle fondamental dans
les résultats réfractifs. L’étroitesse du rapport iris/ICL est à
l’origine de rares complications telles que le blocage pupil-
laire, le syndrome de dispersion pigmentaire, l’uvéite. . .
Keuch et Bleckmann [41] ont rapporté que les cycles de
contraction/dilatation pupillaire, le diamètre pupillaire et
l’amplitude de contraction pupillaire diminuaient après
Figure 12. Cataracte sous-capsulaire antérieure diffuse au neu- l’implantation suggérant une interférence mécanique de
vième mois post-implantation ICL (STAAR Surgical) nécessitant une
l’ICL avec la contraction pupillaire. Mais une étude plus
bilensectomie.
récente de Kamiya [42] portant sur 30 yeux, a démontré
que les diamètres pupillaire d’entrée et pupillaire réel
les techniques photoablatives, permettant une augmenta- diminuaient sensiblement le premier jour postopératoire
tion de la meilleure acuité visuelle corrigée [36]. avant de retrouver leur valeur préopératoire à la première
La perte cellulaire endothéliale centrale atteint à cinq semaine postopératoire, et ce de facon stable jusqu’au
¸
ans 6,4 % du capital préopératoire, soit 3,78 % la première 12e mois postopératoire, en faveur d’une irritation méca-
année principalement expliquée par l’incision cornéenne nique peropératoire et d’une réaction inflammatoire uvéale
peropératoire, puis 0,69 % par an en moyenne jusqu’au postopératoire immédiate. Notre étude n’a pas relevé de
terme du suivi, ce qui correspond à la perte physiologique modification significative du diamètre pupillaire du premier
annuelle admise (0,6 %). La diminution de la densité cellu- au 48e mois postopératoire. Les rares cas de déformation
laire endothéliale varie selon les études : de 3,7 % à quatre pupillaire, d’hyporéactivité pupillaire ou de dispersion pig-
ans pour Kamiya [26], de 6,5 % à deux ans pour Jiménez- mentaire à long terme soulignent l’inocuité mécanique et
Alfaro [16] ou de 8,4 à 9,7 % à trois ans selon l’étude FDA inflammatoire de l’implantation ICL.
[13]. Cette relative inocuité endothéliale s’explique par la Le rétrécissement significatif de l’angle irido-cornéen
biocompatibilité de l’ICL et par le respect d’une distance de d’environ 40 % selon Chung [43] (32 % dans notre étude) est
sécurité moyenne de 2,41 (± 0,23) mm entre l’endothélium stable au-delà du premier mois post-implantation ICL, et ne
central et la face antérieure de l’ICL. Pitault [37] a mesuré s’accompagne pas d’augmentation de la pression intraocu-
par biomicroscopie ultrasonore (UBM) cette même dis- laire ni de la pigmentation trabéculaire. Un suivi rigoureux
tance de sécurité moyenne de 2398 (± 203) m sur 17 cas le premier mois postopératoire est cependant requis dans
d’implantation ICL. La PKR adjuvante pratiquée sur 17 yeux ce contexte.
n’a pas majoré la perte cellulaire endothéliale (− 6,2 %) Selon l’étude américaine FDA [44], l’implantation ICL
confirmant les résultats de Patel [38]. Signalons que le seul torique a fait preuve de son efficacité et de sa prédictibilité
implant phaque de chambre antérieure à appuis angulaires réfractives pour la correction des astigmatismes myopiques
encore disponible, l’implant Acrysof phaque (Alcon® ) ne modérés à forts. Schallhorn et al. [45] ont rapporté la
semble pas induire de majoration de la perte cellulaire supériorité de l’implantation ICL torique sur la PRK en
endothéliale à un an [21]. termes de sécurité, efficacité, reproductibilité et stabilité
Nous avons rapporté cinq cas d’opacification capsulaire réfractives.
antérieure (5,5 %) et trois cataractes cliniquement significa- En conclusion, l’implantation ICL est le traitement de
tives (3,3 %) induits par l’implantation ICL (Fig. 12). Les trois choix pour la correction des amétropies modérées à fortes
cas de cataracte ont concerné des patients de plus de 43 ans, en garantissant d’excellents résultats réfractifs et une
présentant des myopies fortes, et obtenu avec l’implant ICL sécurité stable dans le temps. La quête d’une efficacité
V3 pour un cas (Fig. 9 et 10). Les études de Gonvers [39], et d’une sécurité absolues de l’implantation phaque en
Lackner [12] et Sanders [11] identifient l’âge supérieur à chambre postérieure requiert deux conditions : d’une part,
45 ans, les myopies fortes, le traumatisme peropératoire, et le suivi rapproché et prolongé des patients implantés,
un design et une taille d’implant inadéquats comme des fac- d’autre part, l’accès au 3D sans extrapolation du sulcus
teurs de risque d’opacification capsulaire précoce. Kamiya postérieur — exclusivement accessible par l’échographie 3D
[26] a rapporté une incidence de 1,8 % de cataracte clini- haute fréquence — dans un double objectif : la prétention
quement significative à quatre ans avec l’ICL V4 ; Sanders de l’ajustage sur mesure de la taille de l’implant avec
[13] a rapporté une incidence de cataracte sous-capsulaire simulation préopératoire et l’aide au suivi postopératoire.
antérieure avec les modèles d’ICL V3 et V4 respectivement L’implantation ICL deviendrait alors une alternative à la
de 12,6 % et 2,9 %, probablement en raison du « vault » sup- photoablation cornéenne pour la correction des amétropies
plémentaire de 0,13 à 0,21 mm du modèle V4 par rapport faibles (sous réserve d’un niveau de sécurité et de prédicti-
au V3. Dans notre étude, le « vault » moyen était de 0,52 bilité acquis).
Pour citer cet article : Le Loir M, Cochener B. Résultats à long terme de l’implantation phaque de chambre postérieure
pour la correction des amétropies fortes. J Fr Ophtalmol (2012), doi:10.1016/j.jfo.2011.06.006](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-8-320.jpg)
![Modele +
JFO-489; No. of Pages 10 ARTICLE IN PRESS
Implantation phaque de chambre postérieure pour correction des amétropies fortes 9
Déclaration d’intérêts [19] Lackner B, Pieh S, Schmidinger G, Hanselmayer G, Dejaco-
Ruhswurm I, Funovics MA, et al. Outcome after treatment
Les auteurs déclarent ne pas avoir de conflits d’intérêts en of ametropia with implantable contact lenses. Ophtalmology
2003;110:2153—61.
relation avec cet article.
[20] Pineda-Fernandez A, Jaramillo J, Vargas J, Jaramillo M, Jara-
millo J, Galindez A. Phakic posterior chamber intraocular lens
for high myopia. J Cataract Refarct Surg 2004;30:2277—83.
[21] Kohnen T, Knorz MC, Cochener B, Gerl RH, Arné JL, Colin
Références J, et al. AcrySof Phakic angle-supported intraocular lens for
the correction of moderate to high myopia: one-year results
[1] Azard DT. Intraocular lenses in cataract and refractive surgery. of a multicenter european study. Ophtalmology 2008;115:
Philadelphia: WB Saunders; 2001. 464—72.
[2] Lovisolo CF, Pesando PM. The implantable contact lens. Roma: [22] Gierek-Ciaciura S, Gierek-Lapinska A, Ochalik K, Mrukwa-
Fabiano Editore; 1999. p. 63—72. Kominek E. Correction of high myopia with different phakic
[3] Saragoussi JJ, Arné JL, Colin J, Montard M. Chirurgie refrac- anterior chamber intraocular lenses: ICARE angle-supported
tive. Rapport société francaise d’ophtalmologie. Paris: Masson;
¸ lens and Verisyse iris-claw lens. Graefes Arch Clin Exp Oph-
2001. p. 303—19. talmol 2007;245:1—7.
[4] Baikoff G, Arne JL, Bokobza Y, Colin J, George JL, Lagoutte F, [23] Benedetti S, Casamenti V, Marcaccio L, Brogioni C, Assetto
et al. Angle-fixated anterior chamber phakic intraocular lens V. Correction of myopia of 7 to 24 diopters with the Artisan
for myopia of -7 to -19 diopters. J Refract Surg 1998;14:282—93. phakic intraocular lens: two-year follow-up. J Refract Surg
[5] Benedetti S, Casamenti V, Benedetti M. Long-term endothelial 2005;21:116—26.
changes in phakic eyes after Artisan intraocular lens implanta- [24] De Souza RF, Forseto A, Nosé R, Belfort Jr R, Nosé W. Anterior
tion to correct myopia: five-year study. J Cataract Refract Surg chamber intraocular lens for high myopia: five-year results. J
2007;33:784—90. Catract Refract Surg 2001;27:1248—53.
[6] Mimouni F, Colin J, Koffi V, Bonnet P. Damage to the corneal [25] Perez-Santonja JJ, Alìo JL, Jimenez-Alfaro I, Zato MA. Surgical
endothelium from anterior chamber intraocular lenses in pha- correction of severe myopia with an angle-supported phakic
kic myopic eyes. Refract Corneal Surg 1991;7:277—81. intraocular lens. J Cataract Refract Surg 2000;26:1288—302.
[7] Alió JL, de la Hoz F, Pérez-Santonja JJ, Ruiz-Moreno JM, Que- [26] Kamiya K, Shimizu K, Igarashi A, Hikita F, Komatsu M. Four-
sada JA. Phakic anterior chamber lenses for the correction year follow-up of posterior chamber phakic intraocular lens
of myopia: a 7-year cumulative analysis of complications in implantation for moderate to high myopia. Arch Ophtalmol
263 cases. Ophtalmology 1999;106:458—66. 2009;127:845—50.
[8] Baikoff G, Bourgeon G, Jodai HJ, Fontaine A, Vieira Lellis F, [27] Pesando PM, Ghiringhello MP, Di Meglio G, Fanton G. Posterior
Trinquet L. Pigment dispersion and artisan implants: crystalline chamber phakic intraocular lens for hyperopia: 10-year follow-
lens rise as a safety criterion. J Fr Ophtalmol 2005;28:590—7. up. J Cataract Refract Surg 2007;33:1579—84.
[9] Cochener B. Anterior chamber versus posterior chamber phakic [28] Sanders DR, Vukich JA. Comparison of implantable contact lens
IOLs. J Fr Ophtalmol 2007;30:539—51. and Laser assisted in situ keratomileusis for moderate to high
[10] Stulting RD, John ME, Maloney RK, Assil KK, Arrowsmith PN, myopia. Cornea 2003;22:324—31.
Thompson VM. Three-year resultsof artisan/verisyse phakic [29] Sanders DR, Vukich JA. Comparison of implantable contact lens
intraocular lens implantation results of the United States FDA and Laser assisted in situ keratomileusis for low myopia. Cornea
clinical trial. Ophtalmology 2008;115:464—720. 2006;25:1139—46.
[11] Sanders DR. Anterior subcapsular opacities and cataracts [30] Sanders DR. Matched population comparison of the Visian
5 years after surgery in the visian implantable Collamer lens implantable Collamer lens and standard LASIK for myopia of
FDA trial. J Refract Surg 2008;24:566—70. —3.00 to —7.88 diopters. J Refract Surg 2007;23:537—53.
[12] Lackner B, Pieh S, Schmidinger G, Simader C, Franz C, Dejaco- [31] Awwad ST, Bowman RW, Cavanagh HD, McCulley JP. Wavefont-
Ruhswurm I, Skorpic C. Long-term results of implantation of guided LASIK for myopia using the LADAR custom cornea and
phakic posterior chamber intraocular lenses. J Cataract Refract the VISX custom vue. J Refract Surg 2007;23:26—38.
Surg 2004;30:2269—76. [32] Bahar I, Levinger S, Kremer I. Wavefront-guided LASIK for myo-
[13] Sanders DR, Doney K, Poco M, United States FDA. clinical trial pia with the Technolas 217z: results at 3 years. J Refract Surg
of the implantable Collamer lens for moderate to high myopia: 2007;23:586—90 [discussion 591].
3-year follow-up. Ophtalmology 2004;111:1683—92. [33] Igarashi A, Kamiya K, Shimizu K, Komatsu M. Visual per-
[14] Zalvidar R, Davidorf JM, Oscherow S. Posterior chamber phakic formance after implantable Collamer lens implantation and
intraocular lens for myopia of -8 to -9 diopters. J Refract Surg wavefront-guided laser in situ keratomileusis for high myopia.
1998;14:294—305. Am J Ophtalmol 2009;148:164el—700el. Epub 2009.
[15] Sanders DR, Brown DC, Martin RG, Shepherd J, Deitz MR, De [34] Kamiya K, Shimizu K, Igarashi A, Komatsu M. Comparison of
Luca M. Implantable contact lens for moderate to high myopia: Collamer toric implantable [corrected] contact lens implanta-
phase 1 FDA clinical study with 6-month follow-up. J Cataract tion and wavefront-guided laser in situ keratomileusis for high
Refract Surg 1998;24:607—11. myopic astigmatism. J Cataract Refract Surg 2008;34:1687—93.
[16] Jiménez-Alfaro I, Benìtez del Castillo JM, Garcìa-Feijoò J, [35] Hersh PS, Fry K, Blaker JW. Spherical aberration after laser
Gil de Bernabé JG, Serrano de la Iglesia JM. Safety of pos- in situ keratomileusis and photorefractive keratectomy: cli-
terior chamber phakic intraocular lenses for the correction nical results and theoretical models of etiology. J Cataract
of high myopia: anterior segment changes after posterior Refract Surg 2003;29:2096—104.
chamber phakic intraocular lens implantation. Ophtalmology [36] Yoon G, Macrae S, Williams DR, Cox IG. Causes of spherical
2001;108:90—9. aberrations induced by laser refractive surgery. J Cataract
[17] Jiménez-Alfaro I, Gomez Telleria G, Bueno JL, Puy P. Contrast Refract Surg 2005;31:127—35.
sensitivity after posterior chamber phakic intraocular lens [37] Pitault G, Leboeuf C, Leroux les Jardins S, Auclin F, Chong-Sit
implantation for high myopia. J Refract Surg 2001;17:641—5. D, Baudoin C. Biomicroscopie ultrasonore des implants phaques
[18] Uusitalo RJ, Aine E, Sen NH, Laatikainen L. Implantable contact de chambre postérieure : étude comparative des modèles ICL
lens for high myopia. J Cataract Refract Surg 2002;28:29—36. et PRL. J Fr Ophtalmol 2005;28:1052—7.
Pour citer cet article : Le Loir M, Cochener B. Résultats à long terme de l’implantation phaque de chambre postérieure
pour la correction des amétropies fortes. J Fr Ophtalmol (2012), doi:10.1016/j.jfo.2011.06.006](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-9-320.jpg)
![Modele +
JFO-489; No. of Pages 10 ARTICLE IN PRESS
10 M. Le Loir, B. Cochener
[38] Patel SV, Bourne WM. Corneal endothelial cell loss 9 years [42] Kamiya K, Shimizu k, Igarashi A, Ishikawa H. Evaluation of pupil
after excimer laser keratorefractive surgery. Arch Ophthalmol diameter after posterior chamber phakic intraocular implanta-
2009;127:1423—7. tion. Eye 2010;24:588—94.
[39] Gonvers M, Bornet C, Othenin-Girard P. Implantable contact [43] Chung TY, Park SC, Lee MO, Ahn K. Changes in iridocorneal angle
lens for moderate to high myopia: relationship of vaul- structure and trabecular pigmentation with STAAR implantable
ting to cataract formation. J Cataract Refract Surg 2003;29: Collamer lens during 2 years. J Refract Surg 2009;25:251—8.
918—24. [44] Sanders DR, Schneider D, Martin R, Brown D, Dulaney D, Vukich
[40] Kamiya K, Shimizu K, Kawamorita T. Changes in vaulting and J, et al. Toric implantable Collamer lens for moderate to high
the effect on refraction after phakic posterior chamber intrao- myopic astigmatism. Ophtalmology 2007;114:54—61.
cular lens implantation. J Cataract Refract Surg 2009;35: [45] Schallhorn S, Tanzer D, Sanders DR, Sanders ML. Rando-
1582—6. mized prospective comparison of visian toric implantable
[41] Keuch RJ, Bleckmann H. Pupil diameter changes and reaction Collamer lens and conventional photorefractive keratectomy
after posterior chamber phakic intraocular lens implantation. for moderate to high myopic astigmatism. J Refract Surg
J Cataract Refract Surg 2002;28:2170—2. 2007;23:853—67.
Pour citer cet article : Le Loir M, Cochener B. Résultats à long terme de l’implantation phaque de chambre postérieure
pour la correction des amétropies fortes. J Fr Ophtalmol (2012), doi:10.1016/j.jfo.2011.06.006](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-10-320.jpg)

![182 CORNEAL LASER SURGERY AFTER ICL
ICL implants to correct hyperopia with astigmatism are the manufacturer using a modified vertex formula. The
still not available, and therefore, the pIOL could only ICL surgical procedure was the same as the one previ-
correct the spherical component of the refractive error ously reported by the authors22,23.
and as a result coexisting astigmatic error had to be
treated by either keratorefractive procedure. Combined
Laser surgery
phakic IOL implantation and corneal refractive surgery
was initially described by Zaldivar et al21 who termed LASIK or PRK were performed at least 3 months
the use of LASIK after pIOL implantation bioptics to after ICL surgery and every eye showed a stable refrac-
treat extreme myopia and myopia combined with astig- tion and corneal topographic pattern for at least 3
matism. However, to our knowledge there are no months before performing LASIK or PRK, both sur-
reports on bioptics to treat residual refractive error after geries were carried out by the same surgeon (JFA).
hyperopic ICL. With the present study we assessed the LASIK was performed in 50 eyes and PRK in 12
efficacy and safety results on bioptics with ICL implan- eyes depending on the corneal thickness and ablation
tation to treat hyperopia with astigmatism. depth of each patient.
In the case of myopic astigmatism, ablation was per-
formed in the steepest meridian (negative cylinder abla-
PATIENTS AND METHODS
tion). In the case of mixed astigmatism, half of the abla-
The study population comprised 62 eyes of 35 tion was performed in the steep meridian (negative cylin-
patients who underwent PRK or LASIK for the correc- der ablation) and half in the flat meridian (positive cylin-
tion of residual refractive errors after implantation of a der ablation), the so-called cross-cylinder technique.
Collamer pIOL for hyperopia correction (ICL) at the All surgical procedures were uneventful and with-
Fernández-Vega Ophthalmological Institute (Oviedo, out post-surgical complications within the follow-up
Spain) between February 2005 and April 2009. At the time presented in this study.
time of the surgery, all patients were fully informed of
the details and possible risks of the surgical procedures.
Postoperative Assessment
Written informed consent was obtained from all
patients before surgery in accordance with the Both after pIOL surgery and after LASIK/PRK all
Declaration of Helsinki and an institutional review the patients fulfilled the follow-up protocol in which the
board approved the study. examination visits were carried out at Day 1, Week 1,
The inclusion criteria for ICL implantation were cor- and Month 1, and then every 3 months as necessary.
rected distance visual acuity (CDVA) of 20/50 or better, Data obtained in each postoperative follow-up visit
stable refraction and clear central cornea. The exclusion included uncorrected distance visual acuity (UDVA),
criteria included age <22 years, anterior chamber depth CDVA, slit-lamp examination, refraction, ECD, fundus
<2.8 mm, endothelial cell density (ECD) examination, intra-ocular pressure (IOP) and central
<2000 cell/mm2, cataract, history of glaucoma or retinal separation between the lens anterior surface and the pos-
detachment, macular degeneration or retinopathy, terior surface of the ICL (Vault). For averaging, visual
neuro-ophthalmic diseases and history of ocular inflam- acuities were converted to logMAR values; then, the
mation. Before the ICL implantation, patients had a means and standard deviations were back calculated to
complete ophthalmologic examination, including mani- Snellen acuity. Sphero-cylindrical refractive results were
fest and cycloplegic refraction, keratometry, corneal converted into vectors expressed by three dioptric pow-
topography and pachymetry using the Orbscan II ers: M, J0, and J45; with M being equal to the spherical
(Bausch & Lomb, Rochester, NY), ECD (SP 3000P; equivalent (SE) of the given refractive error, and J0 and
Topcon Europe Medical, Netherlands), slit-lamp exami- J45 the two Jackson crossed cylinders equivalent to the
nation, Goldmann aplanation tonometry and binocular conventional cylinder. Manifest refractions in conven-
indirect ophthalmoscopy through dilated pupils. tional script notation (S [sphere], C [cylinder], · [axis])
were converted to power vector coordinates and overall
blurring strength using the formulas described by
ICL size and power calculation
Thibos and Horner24: M = S+C/2; J0 = (–C/2)*cos (2α);
All eyes were implanted with a model ICHV3 J45 = (–C/2)* sin (2α) and B = (M2 + J02+ J452)1/2.
(STAAR Surgical, Nidau, Switzerland). The ICL size Data analysis was performed using SPSS for
was individually determined based on the horizontal Windows version 16.01 (SPSS Inc. Chicago. IL).
white-to-white distance and anterior chamber depth Normality of data was checked by Kolmogorov-
(ACD) measured with Orbscan II (Bausch & Lomb. Smirnov test and analyzed using the Wilcoxon rank
Rochester, NY) following the manufacturer’s recom- sum test, or analysis of variance with multiple compar-
mendations. Power calculation for the ICL was per- isons correction where appropriate, to explore statisti-
formed using the software ICL power table provided by cal differences for refractive and visual acuity scores
JOURNAL OF EMMETROPIA - VOL 2, OCTOBER-DECEMBER](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-12-320.jpg)
![CORNEAL LASER SURGERY AFTER ICL 183
among different follow-up visits. Bivariate correlations Table 1. Descriptive statistics for demographic data of
between attempted versus achieved refraction were ana- patients and characteristics of implanted Hyperopic
lyzed using a non-parametric (Spearman’s coefficient) Implantable Collamer Lens
correlation analysis. Differences were considered to be
statistically significant when the p value was <0.05. Range
Mean SD
[Min, Max]
RESULTS Age (years) 27.6 4.3 [20,40]
Refractive sphere (D) 5.73 1.79 [1.50,11.00]
The mean age of the 35 patients, 19 women Refractive cylinder (D) –2.07 1.03 [–4.00,0.00]
(54.3%) and 16 men (45.7%), was 27.6 years ± 4.3 Flat keratometry 41.2 1.9 [36.5,45.8]
(SD) (range 20 to 40 years). The mean interval Steep keratometry 43.3 2.0 [39.0,47.8]
between ICL surgery and LASIK /PRK was 4.9± 3.9 ICL size (mm) 12.00 0.30 [11.5,12.5]
months (range 3 to 19 months). Fifty-one eyes had ICL sphere (D) 8.4 2.7 [3.0,14.0]
residual myopia or myopic astigmatism, 11 eyes had ECC (cells/mm2) 2775 313 [2105,3377]
White-White (mm) 11.9 0.4 [11.0,12.9]
mixed astigmatism after ICL surgery. Mean follow-up ACD (mm) 3.0 0.2 [2.8,3.4]
after laser treatment was 9.7±7.4 months (range 3 to CCT (µm) 538 54 [410,640]
27 months). Table 1 shows the preoperative patient
demographics and ICL characteristics. D: diopters; ICL: Implantable Collamer Lens; ACD: anterior
chamber depth; ECC: endothelial cell count; CCT: central corneal
thickness.
Refractive outcomes
The overall change in manifest refraction is shown ifest refractive sphere was 5.73±1.79 D (range 1.50 to
in Figure 1. Prior to ICL implantation, the mean man- 11.00 D) and the mean manifest refractive cylinder was
–2.07±1.03 (range –4.00 to 0.00 D). At the latest fol-
low-up visit following laser treatment the mean mani-
fest refractive sphere was –0.01±0.08 (range –0.50 to
0.25 D) and manifest refractive cylinder was
–0.19±0.36 (range –1.50 to 0.00 D). The distribution
of the refractive components after vector conversion
before and after the different laser treatments is shown
in table 2. No statistically significant differences existed
in the M, J0 or J45 components among patients under-
going either laser procedure. The power vector magni-
tude was reduced either after ICL surgery or after differ-
ent laser treatments and the mean value in all compo-
nents of refraction after laser surgery were neither clini-
cally nor statistically significant between the different
laser procedures (P>0.05, Kruskal-Wallis test for all vec-
tor components of refraction). Figure 2 shows the astig-
Figure 1. Time course of the Manifest refractive sphere (MRSE)
matic components of the power vector as represented by
and cylinder (MRCYL) in diopters (D) after laser surgery. the 2-dimensional vector plot (J0, J45). The dispersion
Table 2. Mean values and standard deviation (SD) of components of vectorial decomposition of refraction before and at different
follow-up times after surgery
Pre-operatively Pos ICL Pos Laser
M J0 J45 M J0 J45 M J0 J45
Mean±SD Mean±SD Mean±SD Mean±SD Mean±SD Mean±SD Mean±SD Mean±SD Mean±SD
LASIK 4.9±1.6 0.7±0.7 -0.1±0.5 -0.8±0.7 0.5±0.5 -0.1±0.4 -0.1±0.2 0.1±0.2 0.0±0.1
PRK 3.8±2.0 0.9±0.6 -0.1±0.7 -1.1±0.6 0.7±0.6 -0.1±0.5 -0.1±0.1 0.1±0.1 0.0±0.0
p* 0.085 0.755 0.914 0.211 0.880 0.928 0.896 0.742 0.727
SD: Standard deviation.
* Independent-Samples Kruskall-Wallis Test.
JOURNAL OF EMMETROPIA - VOL 2, OCTOBER-DECEMBER](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-13-320.jpg)




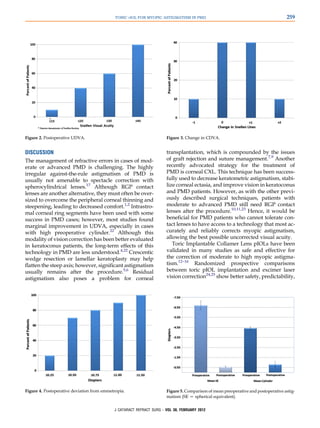
![TORIC PIOL FOR MYOPIC ASTIGMATISM IN PMD 257
Co.) has been used to safely and reliably correct of PMD was based on these data, analysis of corneal topog-
moderate to high myopia and astigmatism.12–16 Given raphy indices by the Corneal Navigator feature of the OPD
Scan II, and clinical judgment. All patients had a high index
that the progression of corneal thinning and ectasia in
of suspicion (above 90%) for PMD as indicated by the
PMD, like keratoconus, tends to stabilize in the third or Corneal Navigator. Figure 1 shows the corneal topography
fourth decade of life,17 toric pIOL implantation may be of 1 patient.
another option for the surgical correction of myopic
astigmatism in this setting. Studies18–20 are beginning
Surgical Technique
to show the safety and efficacy of this treatment
modality in keratoconus. To our knowledge, there is All eyes had implantation of an Implantable Collamer
Lens toric pIOL, which is currently approved in Canada
only 1 case report of a patient with PMD who derived for the correction of myopia between À4.00 D and
benefit from toric pIOL implantation.21 This retrospec- À20.00 D and astigmatism between 1.00 D and 4.00 D. Before
tive case series evaluated patients with myopic surgery, all patients had 2 neodymium:YAG peripheral
astigmatism secondary to PMD who had implantation iridotomies in each eye to prevent pupillary block glaucoma.
of the toric pIOL. The horizontal meridian was marked preoperatively at the
slitlamp to account for posture-related ocular cyclotorsion.
Topical anesthesia of bupivacaine (Marcaine 0.75%) was
PATIENTS AND METHODS administered, and the eye was prepared and draped in the
All consecutive cases with PMD that had implantation of standard sterile fashion. Two limbal paracenteses were
the toric pIOL by the same surgeon (H.V.G.) from January created at 6 o’clock and 12 o’clock. Intracameral
1, 2002, to May 30, 2011, were retrospectively reviewed for preservative-free lidocaine 1.00% was injected, and the
postoperative outcomes. Most patients initially presented anterior chamber was filled with hydroxypropyl methylcel-
for consultation regarding corneal refractive surgery due lulose (Ocucoat). A temporal clear corneal incision was
to contact lens intolerance. Eligibility for toric pIOL implan- created with a 2.75 mm diamond keratome blade (Alcon
tation was determined on an individual basis. Stability in Laboratories, Inc.).
the manifest refraction (within G0.50 diopter [D]) in the Next, the toric pIOL was implanted in the eye using an
year before surgery was required. Exclusion criteria in- MSI-TR injector (Staar Surgical Co.) and allowed to unfold.
cluded previous ocular surgery, trauma, amblyopia, ante- The haptics were gently maneuvered into the ciliary sulcus
rior segment pathology other than PMD, posterior using 2 Pallikaris manipulators (Duckworth & Kent Ltd.)
segment pathology other than myopia, and anterior cham- in a hand-over-hand technique. The toric pIOL was gently
ber depth (ACD) less than 2.70 mm. Informed consent rotated into the orientation specified by the manufacturer
was obtained after detailed discussion of all relevant risks, to correct the astigmatism. The 11 o’clock peripheral iridoto-
benefits, and alternatives of the procedure. In particular, my was entered and stretched with a Pallikaris manipulator
patients were informed about the paucity of literature to confirm patency. After the ophthalmic viscosurgical
regarding the use of the toric pIOL in patients with PMD. device was irrigated from the anterior chamber, care was
After surgery, patients were invited to complete a short taken to ensure that the orientation of the toric pIOL had
survey detailing the quality of their distance and night not shifted.
vision; the presence of glare, halos, image ghosting, or Stromal hydration was performed to achieve wound
double vision; other symptoms; and overall satisfaction integrity, and a small bolus of intracameral vancomycin
with the procedure. (1 mg in 0.1 mL of sterile balanced salt solution) was admin-
istered through 1 of the paracenteses. At the end of the
surgery, a drop of apraclonidine 0.5% (Iopidine) and 2 drops
Preoperative Evaluation of ofloxacin (Ocuflox) were given. The same surgical proto-
All eyes had a comprehensive preoperative ophthalmic col was followed in the fellow eye on the same day or 1 or
examination that included corrected distance visual acuity 2 days later.
(CDVA), manifest refraction by autorefraction (Canon,
RK-F1), keratometry, ACD, corneal topography by OPD
Postoperative Evaluation
Scan II (ARK 10000, Nidek Co. Ltd.) and Orbscan IIz (Bausch
& Lomb), and axial length by partial coherence interferome- All eyes were examined postoperatively at 1 day, 1 week,
try (IOLMaster, version 5, Carl Zeiss Meditec AG). Diagnosis and 1, 3, and 6 months. The examinations included UDVA,
CDVA, manifest refraction, intraocular pressure, and pIOL
vaulting. Outcome measures were recorded at the last post-
Submitted: June 6, 2011. operative visit and included UDVA, CDVA, manifest refrac-
Final revision submitted: August 12, 2011. tion, and corneal topography performed using the same
Accepted: August 14, 2011. devices as preoperatively.
From Gimbel Eye Centre (Camoriano, Aman-Ullah, Purba, Sun,
Gimbel) and the University of Calgary (Camoriano, Gimbel), Statistical Analysis
Calgary, Alberta, Canada; Loma Linda University (Gimbel), Loma The mean and standard error of the mean (SEM) were
Linda, California, USA. calculated for the following variables: age, preoperative
CDVA (expressed as the logMAR), postoperative CDVA,
Corresponding author: Howard V. Gimbel, MD, MPH, Gimbel Eye preoperative spherical equivalent (SE), postoperative SE,
Centre, 450, 4935 - 40 Avenue Northwest, Calgary, Alberta T3A and postoperative UDVA. Histograms showing the percent-
2N1, Canada. E-mail: cgy-info@gimbel.com. age of patients achieving a particular level of UDVA, change
J CATARACT REFRACT SURG - VOL 38, FEBRUARY 2012](https://image.slidesharecdn.com/fulltextpdfsfeb2012public1-120308060153-phpapp02/85/Visian-ICL-article-19-320.jpg)