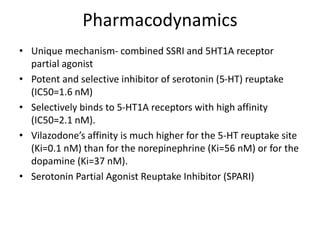
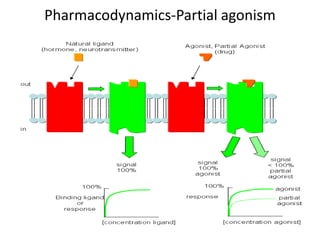
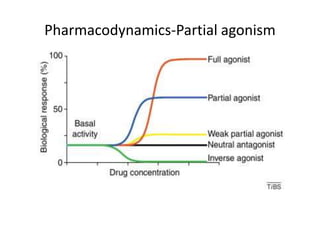
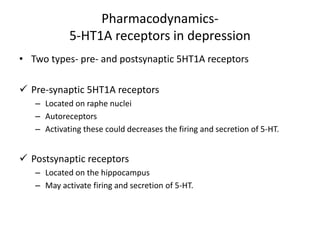
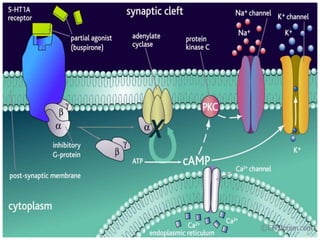
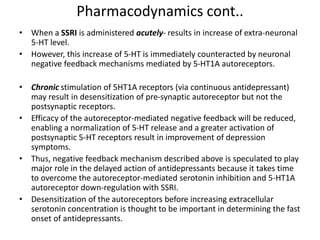
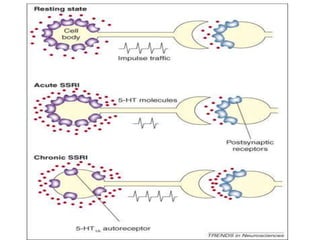
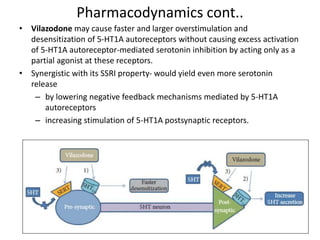
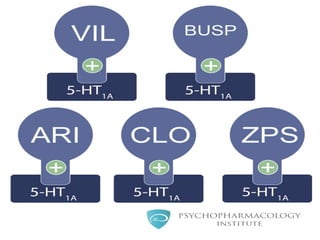
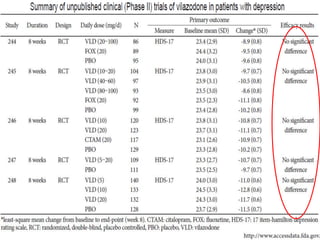
Vilazodone is an antidepressant approved for treatment of major depressive disorder in adults. It is a serotonin partial agonist and reuptake inhibitor (SPARI) with a novel mechanism of action. Vilazodone was originally developed by Merck KGaA and approved by the FDA in 2011 after being licensed to multiple companies. It has shown effectiveness in clinical trials with a side effect profile that is generally milder than SSRIs alone. Vilazodone offers a new option for treatment of depression through its combined serotonergic and partial 5-HT1A agonistic mechanisms of action.




















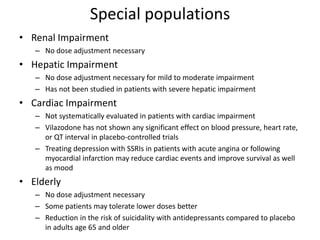
![Special populations cont..
• Children and Adolescents
– Safety and efficacy have not been established
– Use with caution, observing for activation of known or unknown
bipolar disorder and/ or suicidal ideation, and strongly consider
• Pregnancy
– Risk Category C [some animal studies have shown adverse effects; no
controlled studies in humans]
– Not generally recommended for use during pregnancy, especially
during first trimester
– Nonetheless, continuous treatment during pregnancy may be
necessary and has not been proven to be harmful to the fetus
– Must weigh the risk of treatment (first trimester fetal development,
third trimester newborn delivery) to the child against the risk of no
treatment (recurrence of depression, maternal health, infant bonding)
to the mother and child](https://image.slidesharecdn.com/mypptvilazodone-151120190709-lva1-app6892/85/Vilazodone-38-320.jpg)


![References
• Essential psychopharmacology Prescriber’s guide- Stephen M. Stahl. 5th
ed.
• Stahl’s essential psychopharmacology : neuroscientific basis and practical
application / Stephen M. Stahl– 4th ed.
• Rickels K, Athanasiou M, Robinson D, et al. Evidence for efficacy and
tolerability of vilazodone in the treatment of major depressive disorder: a
randomized, double-blind, placebocontrolled trial. J Clin Psychiatry
2009;e1–e8.
• Wang, SM; Han, C; Lee, SJ; Patkar, AA; Masand, PS; Pae, CU (August 2013).
"A review of current evidence for vilazodone in major depressive
disorder.". International Journal of Psychiatry in Clinical Practice 17 (3):
160–9.
• "VIIBRYD (vilazodone hydrochloride) tablet VIIBRYD (vilazodone
hydrochloride) kit [Forest Laboratories, Inc.]". DailyMed. Forest
Laboratories, Inc. December 2012.](https://image.slidesharecdn.com/mypptvilazodone-151120190709-lva1-app6892/85/Vilazodone-41-320.jpg)