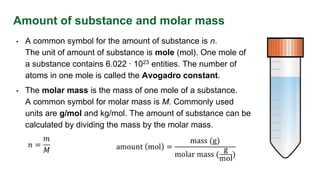
This document provides an overview of key units and concepts used in the International System of Units (SI) including:
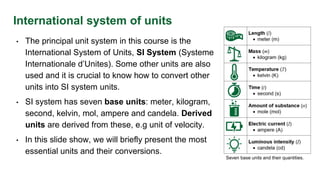
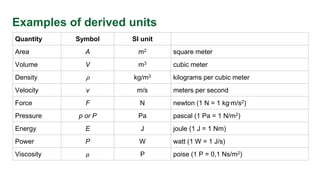
- The seven base SI units of the meter, kilogram, second, kelvin, mole, ampere, and candela. Derived units are derived from base units.
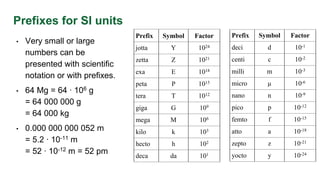
- Prefixes used for very small or large units like kilo, milli, micro, and nano to express units concisely.
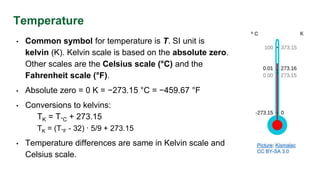
- Conversion of temperature units between kelvin, celsius and fahrenheit scales.
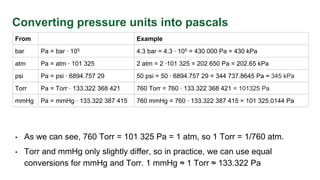
- Conversion of pressure units between pascals, atmospheres, bars, psi, torr and mmHg. Gauge pressure indicates difference from atmosphere.
- Vapor pressure, which indicates how