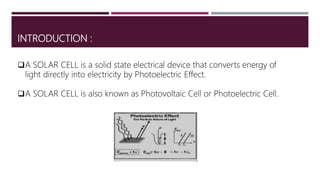
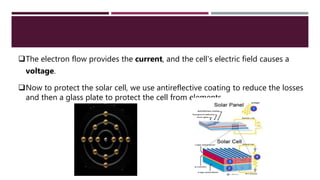
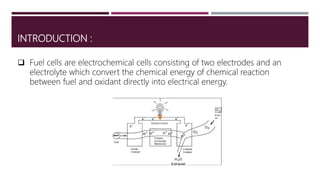
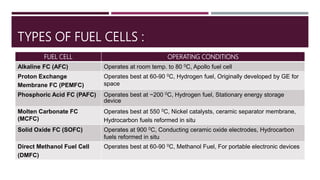
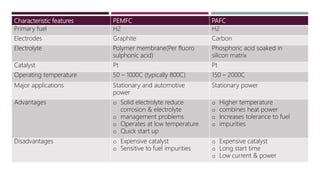
The document provides an overview of solar cells and fuel cells, detailing their construction, function, and applications. Solar cells convert light energy into electrical energy using semiconductors like silicon, while fuel cells generate electricity through electrochemical reactions involving hydrogen and oxygen. Both technologies are presented as sustainable energy solutions with various applications, including rural electrification and powering vehicles.