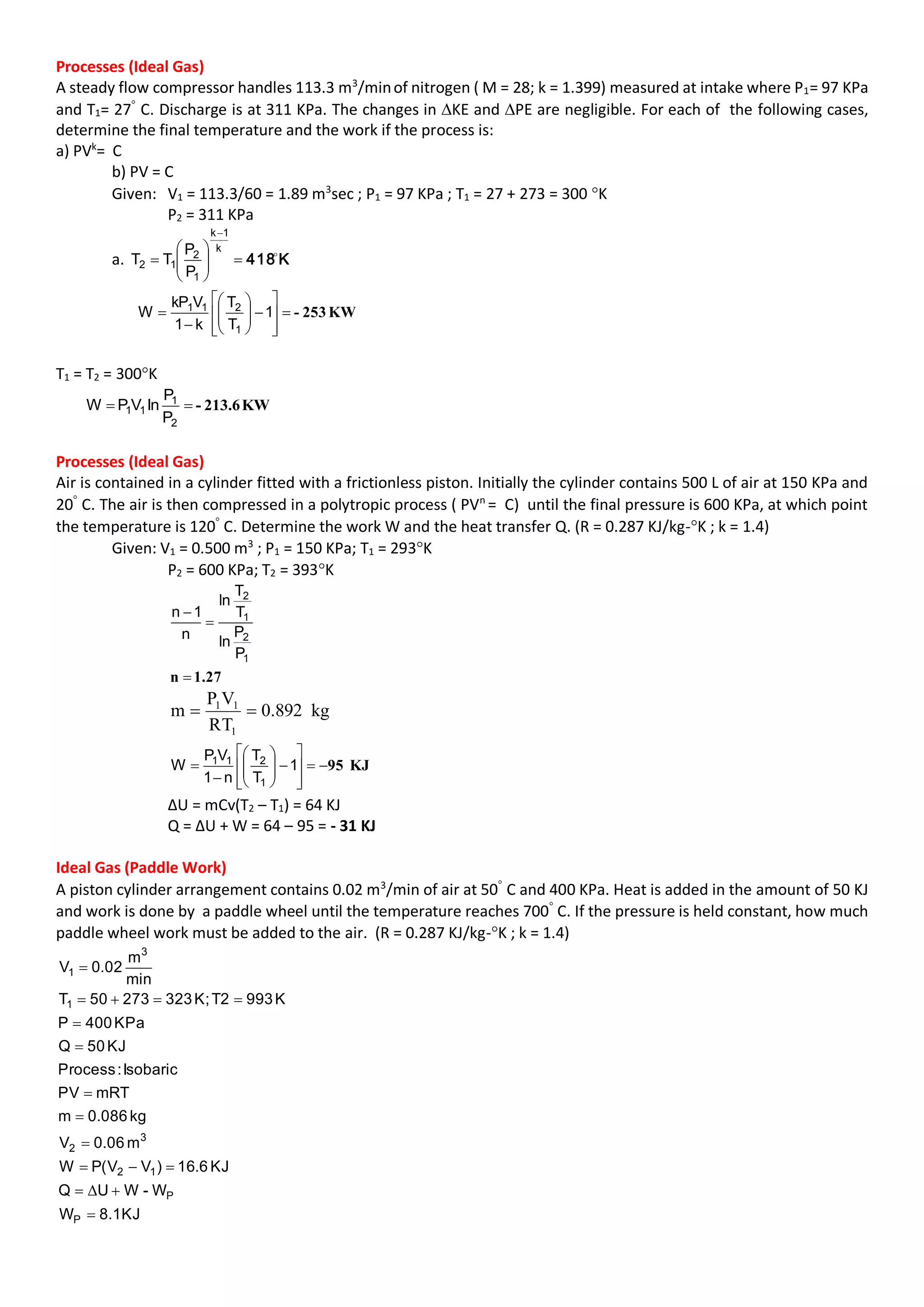
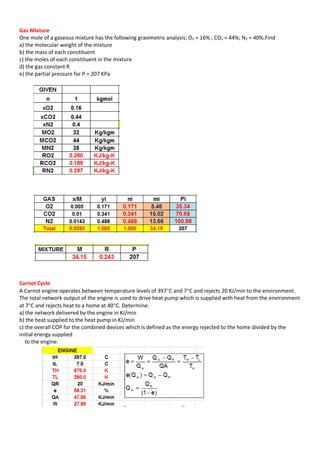
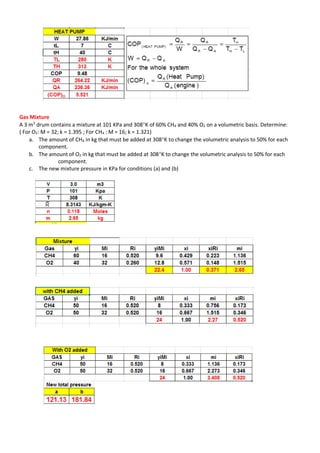
This document contains multiple problems involving ideal gas processes. The first problem describes a steady flow compressor handling nitrogen with known intake conditions and discharge pressure. It asks to determine the final temperature and work for two process types. The second problem involves air in a cylinder being compressed in a polytropic process with known initial and final pressures and temperatures. It asks to determine the work and heat transfer. The third problem describes a gas turbine expanding helium polytropically and asks to determine the final pressure, power produced, heat loss, and entropy change.