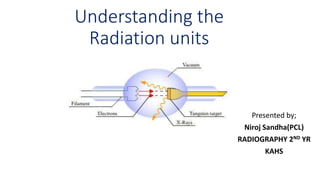
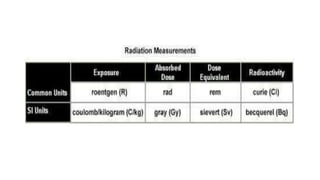
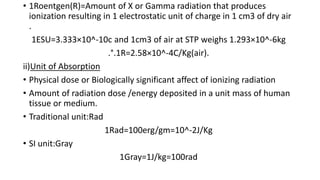
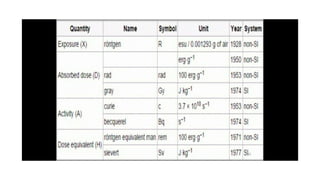
This document discusses various units used to measure radiation and its effects. It introduces electromagnetic radiation and defines ionizing and non-ionizing radiation. It then explains the importance of measurement units and defines common units like becquerels, curies, grays, rads, sieverts and rems used to quantify radioactivity, radiation dose, and biological dose equivalents. The document also discusses concepts like kerma, equivalent dose and effective dose which account for different tissue sensitivities to different radiation types.