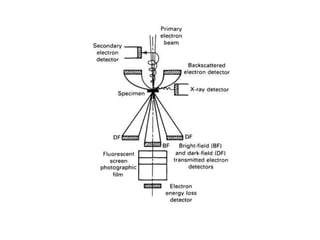
The transmission electron microscope can be used to image microstructural features at high magnifications, perform elemental analysis, and determine crystal structures. Samples must be thinly sectioned or ion milled to be electron transparent. Imaging techniques like bright field and dark field are used to reveal structural features based on diffraction contrast. Selected area diffraction patterns can be indexed to identify crystal structures and orientations. The transmission electron microscope thus provides valuable microscopic and crystallographic information about materials at high resolution.













![Diffraction
Symmetry of diffraction pattern reflects
symmetry of crystal around beam direction
Why does 3-fold diffraction pattern look hexagonal?
[111] in cubic [001] in hexagonal
Example:
6-fold in hexagonal, 3-fold in cubic](https://image.slidesharecdn.com/transmissionelectronmicroscopelecture1-240318172548-76c8fc76/85/Transmission-Electron-Microscope_Lecture1-pptx-15-320.jpg)
![Diffraction
Note: all diffraction patterns
are centrosymmetric,
even crystal structure is not
centrosymmetric (Friedel's
law)
Some 0-level patterns thus
exhibit higher rotational
symmetry than structure has
P cubic reciprocal lattice
layers along [111] direction
0-level
l = +1 level
l = -1 level](https://image.slidesharecdn.com/transmissionelectronmicroscopelecture1-240318172548-76c8fc76/85/Transmission-Electron-Microscope_Lecture1-pptx-16-320.jpg)



![Indexing electron diffraction patterns
Index other reflections by vector sums, differences
Next find zone axis from cross product of any two (hkl)s
(202) x (220) ——> [444] ——> [111]](https://image.slidesharecdn.com/transmissionelectronmicroscopelecture1-240318172548-76c8fc76/85/Transmission-Electron-Microscope_Lecture1-pptx-20-320.jpg)














