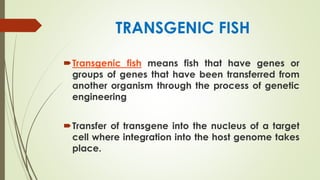
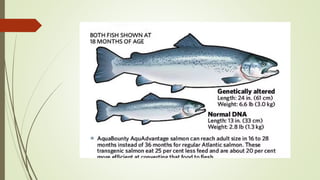
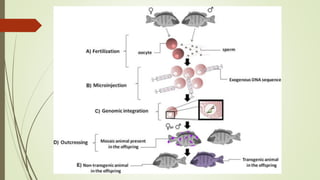
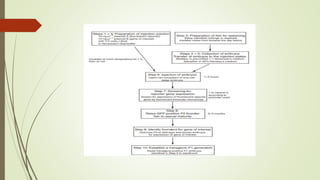
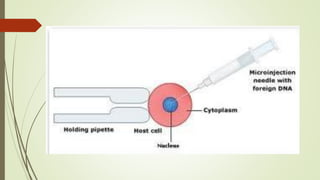
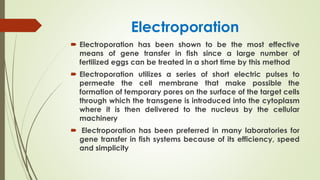
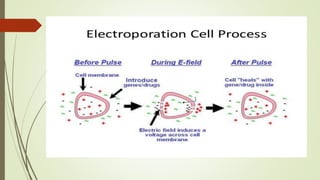
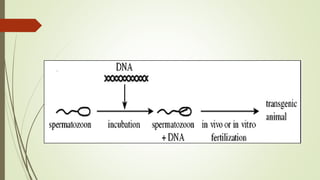
Transgenic fish are genetically modified organisms created through the transfer of genes to enhance growth rates, environmental tolerance, and disease resistance. The U.S. FDA has approved genetically modified fish like GloFish and AquaBounty salmon, though commercialization remains controversial. Various gene transfer methods exist, such as microinjection and electroporation, but potential ecological risks and health concerns persist regarding their release into the environment.