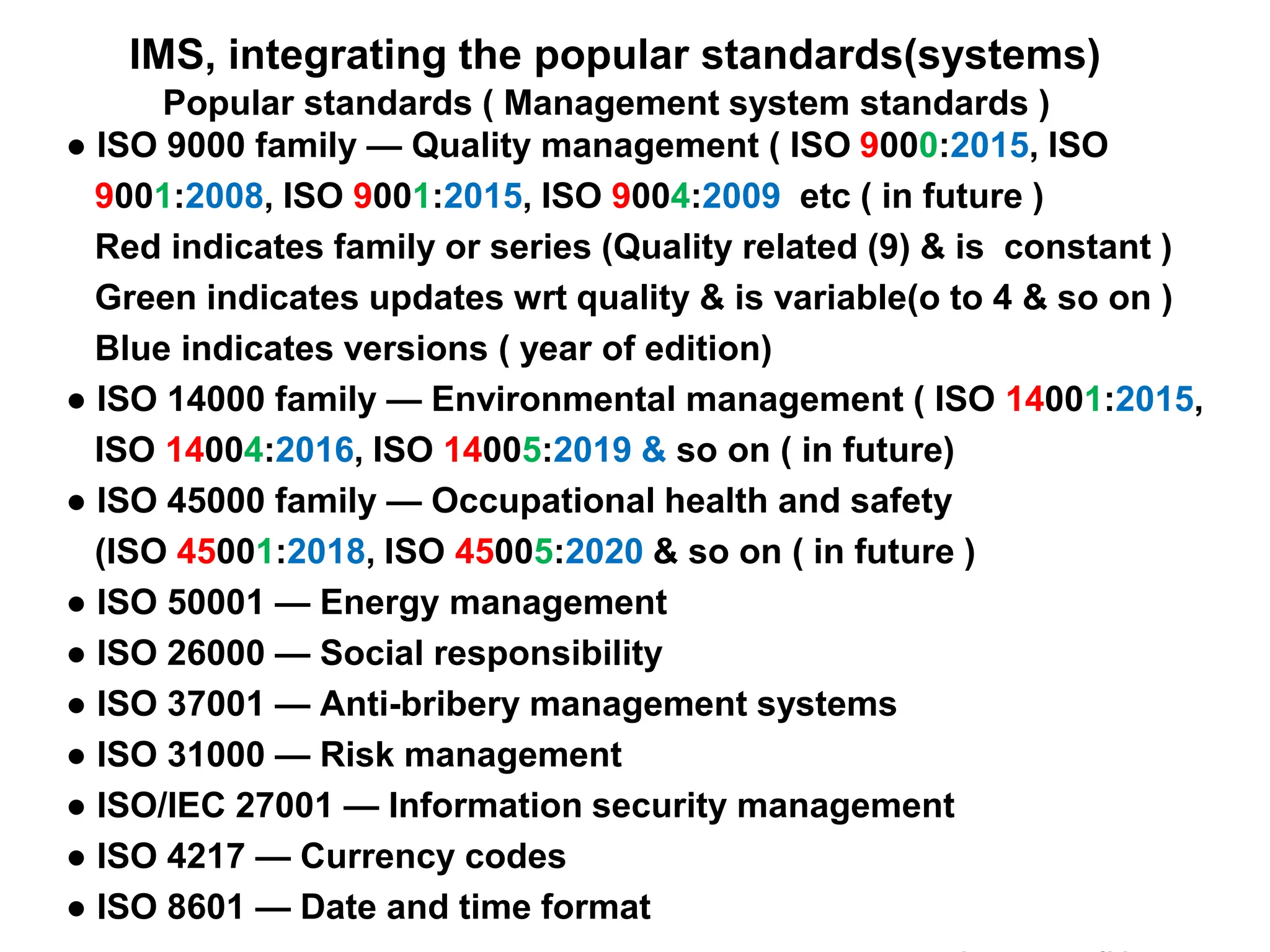
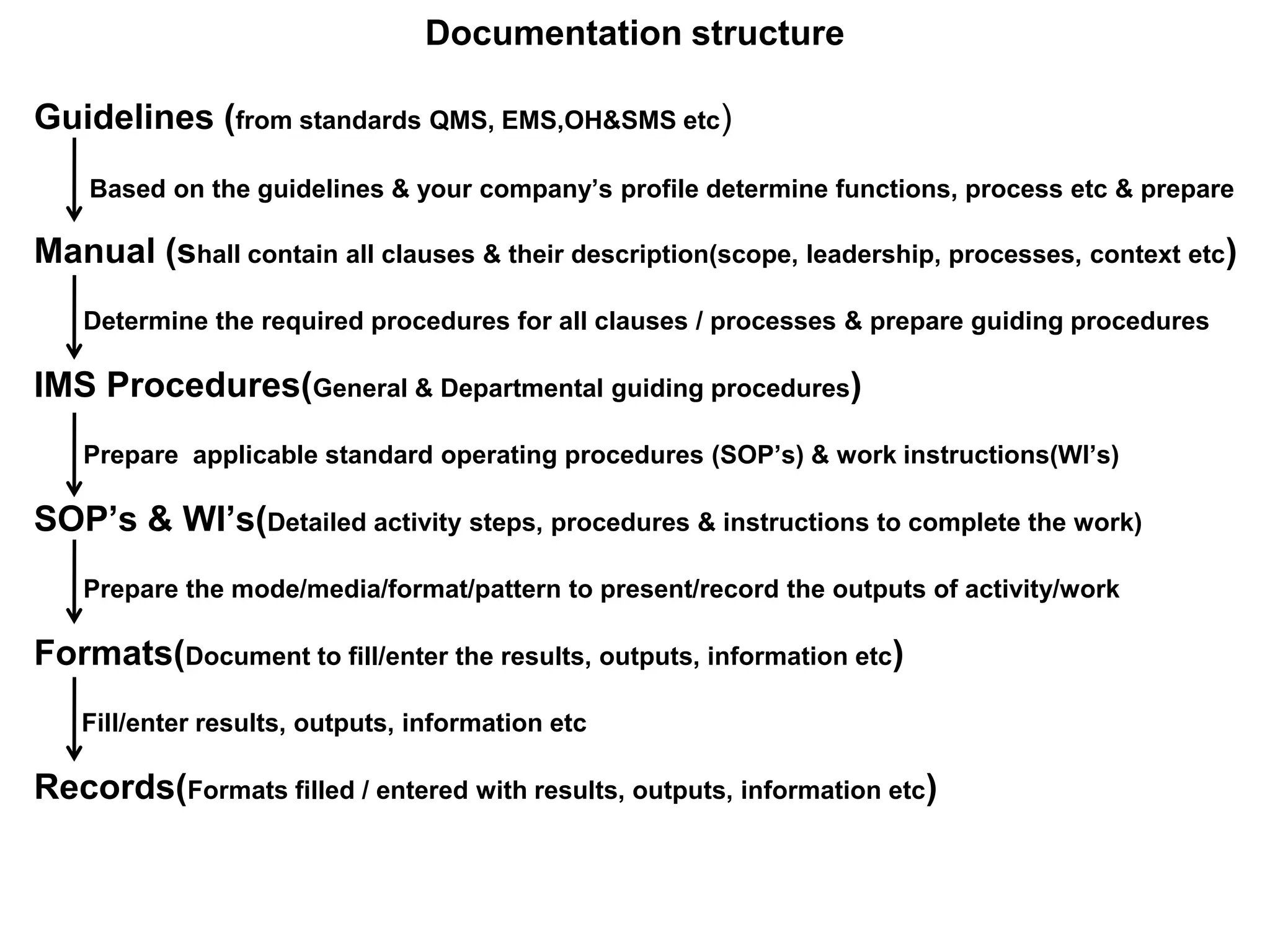
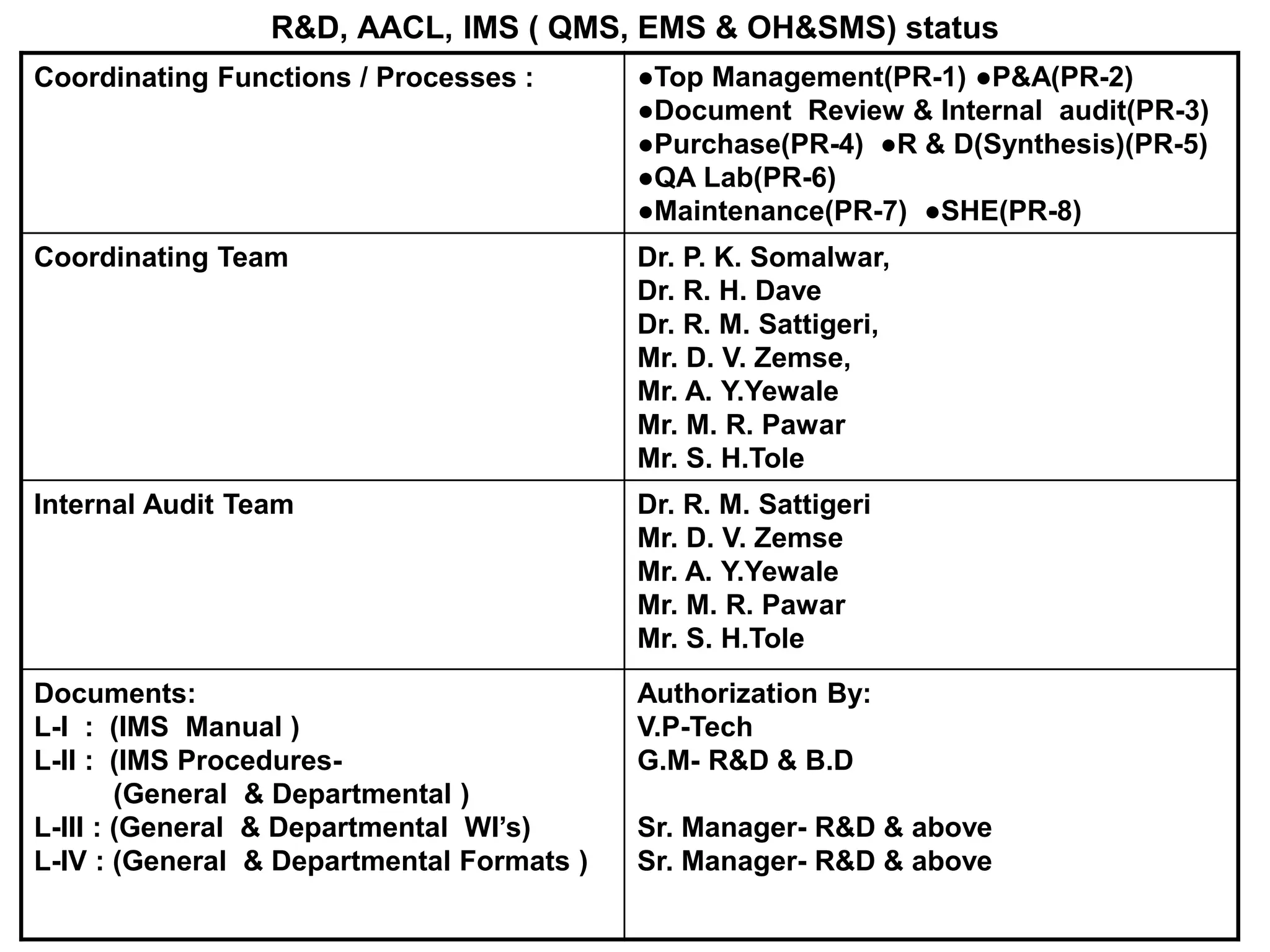
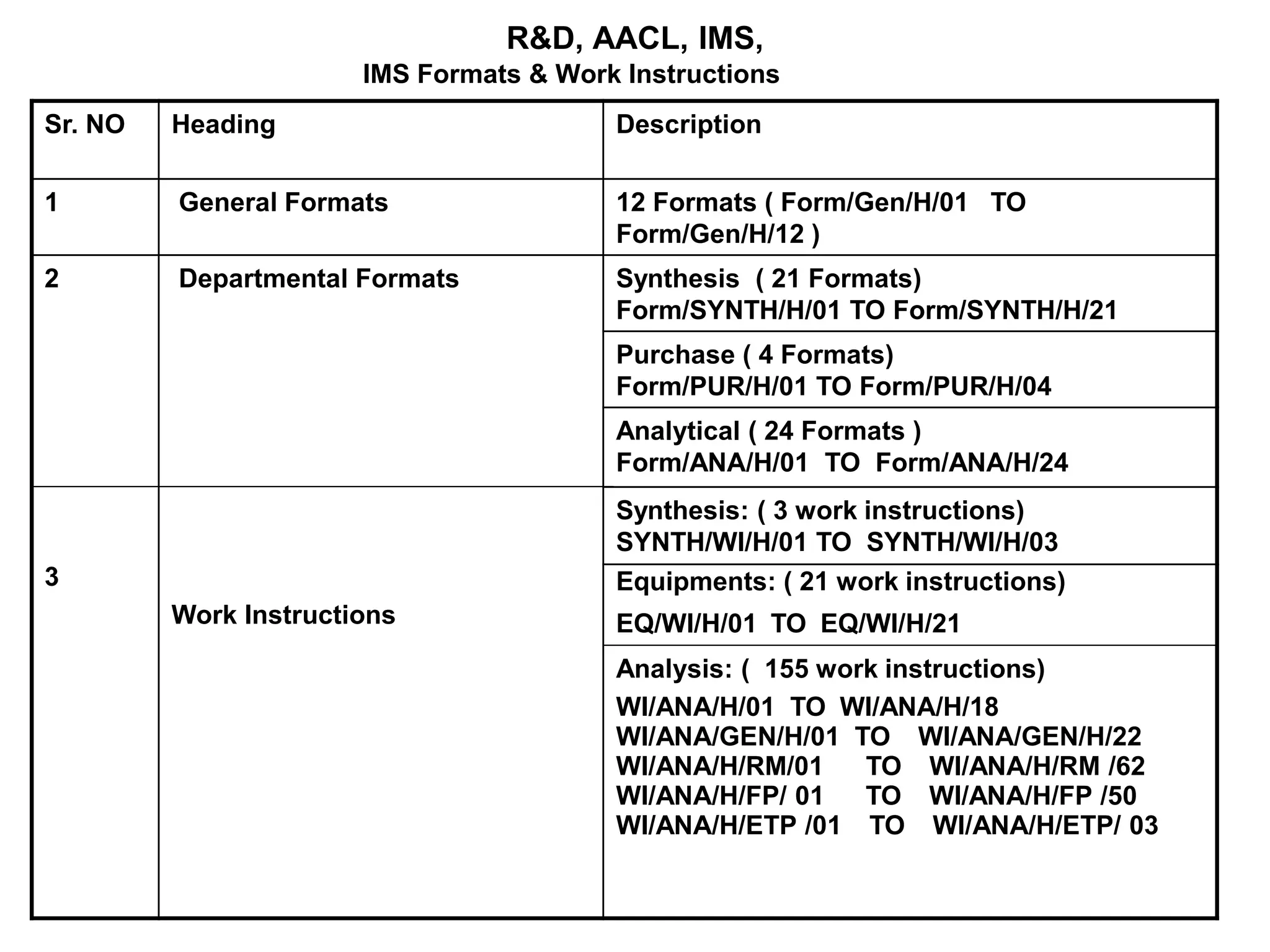
The document outlines the structure and benefits of Integrated Management Systems (IMS) which harmonizes multiple ISO standards, including ISO 9000 (Quality), ISO 14000 (Environmental), and ISO 45000 (Occupational Health and Safety), among others. It details the certification process, the roles of various accreditation bodies, and the historical context of IMS development. Additionally, it discusses the advantages of implementing IMS, such as improved performance and reduced redundancy, alongside a comprehensive review of relevant documentation and procedures.