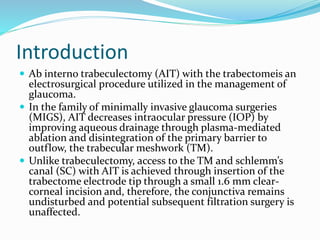
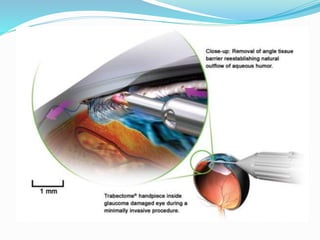
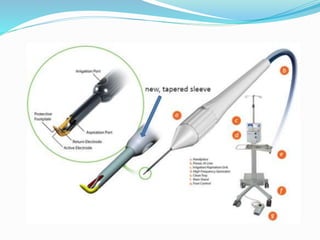
Ab interno trabeculectomy (AIT) using the trabectome is a minimally invasive surgical technique for managing glaucoma by improving aqueous drainage and reducing intraocular pressure. The procedure requires only a small incision, preserving the conjunctiva, and features a favorable safety profile compared to traditional methods. Developed in the early 2000s, the trabectome has gained FDA clearance and is used for treating both adult and infantile glaucoma.