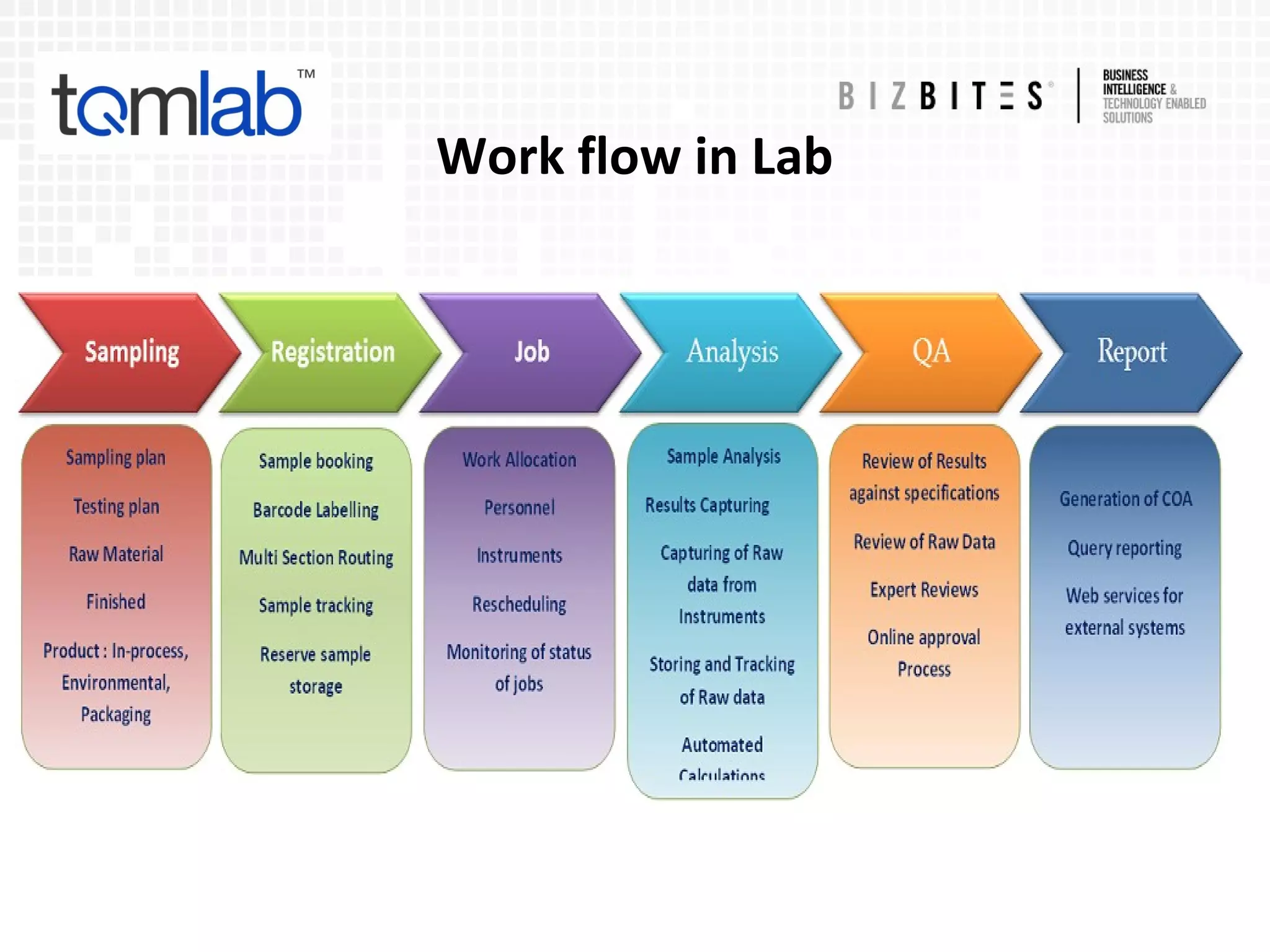
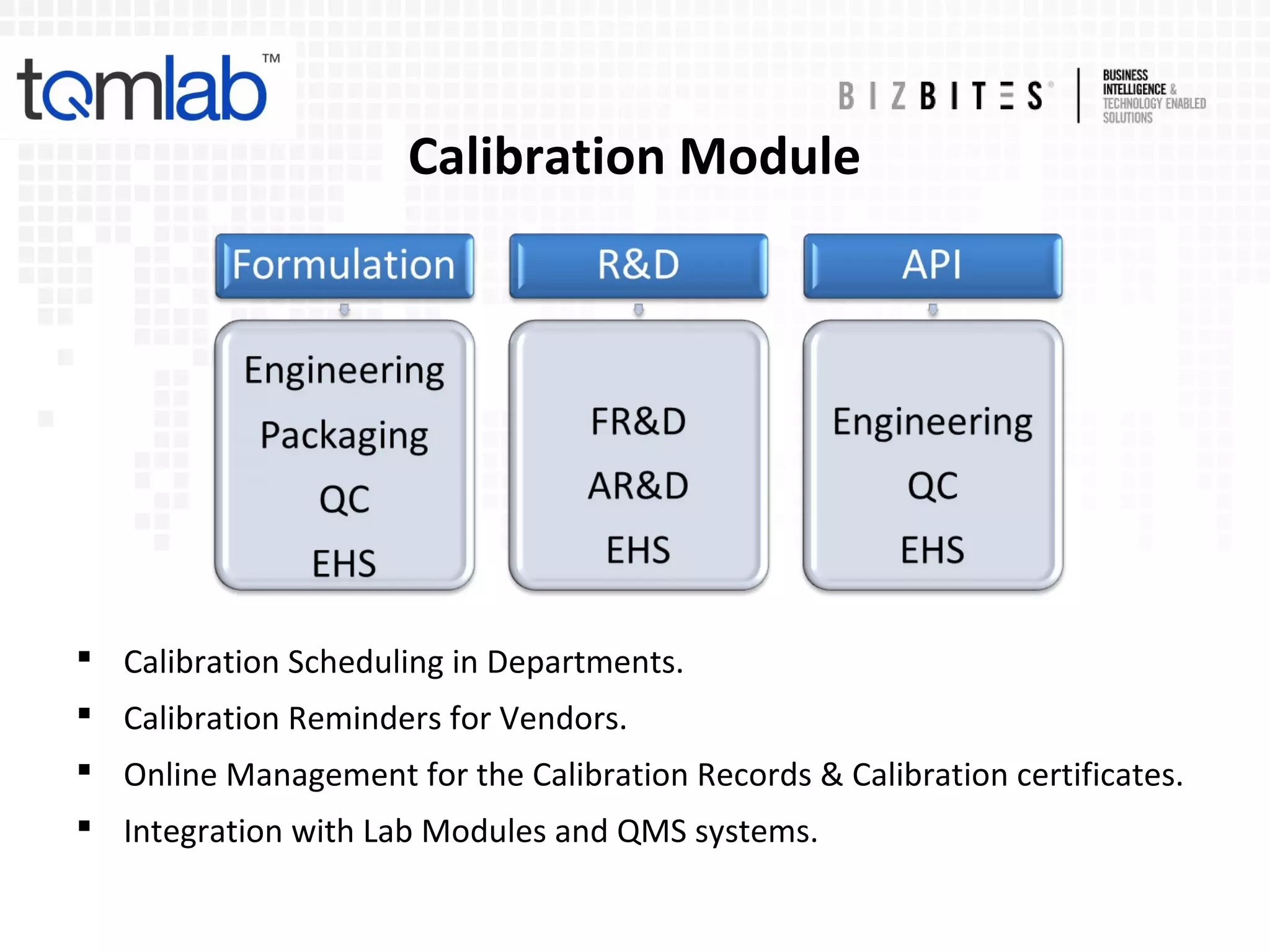
The document outlines a comprehensive laboratory automation solution that enhances efficiency, productivity, accuracy, and security in various sectors such as pharmaceuticals, diagnostics, and food industries. It highlights features such as compliance with regulations, laboratory management functionalities, document management, and health management systems. Additionally, the document discusses advantages for analysts and lab managers, including streamlined operations and reduced administrative burdens.