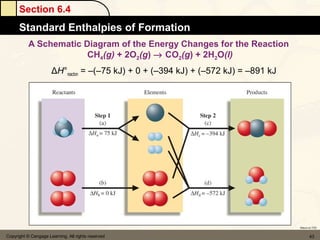
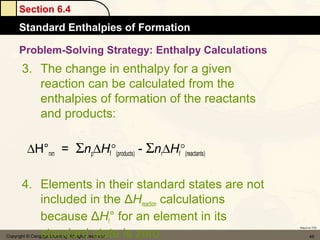
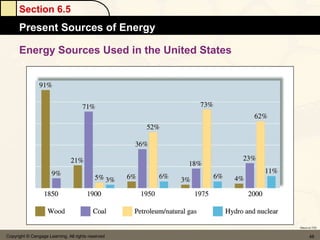
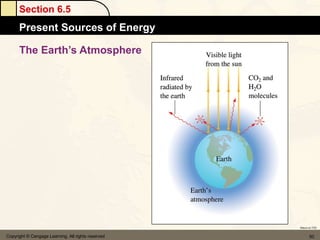
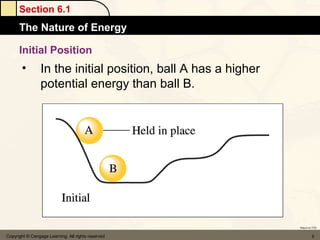
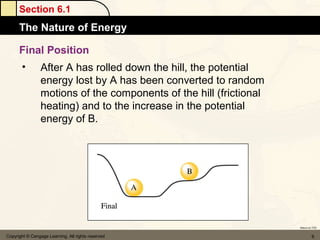
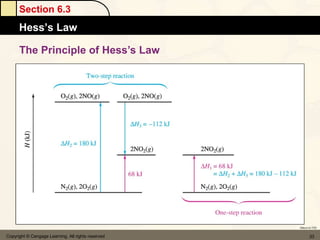
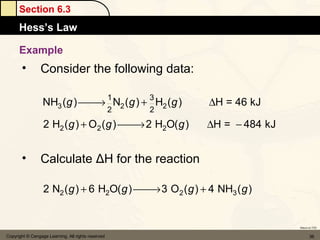
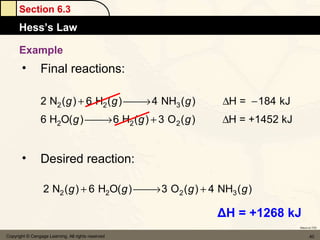
Here are the signs of ΔE for each process:
a) If an endothermic process performs work, and the magnitude of work is greater than the magnitude of heat, then ΔE is negative.
b) If work is done on a gas and the process is exothermic, then ΔE is positive.

















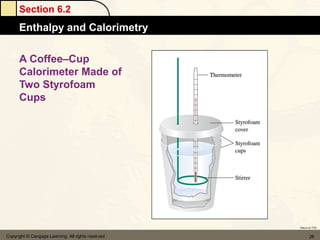
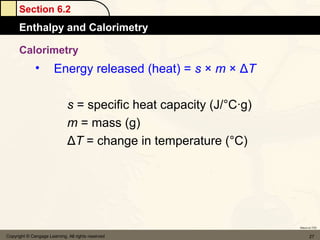

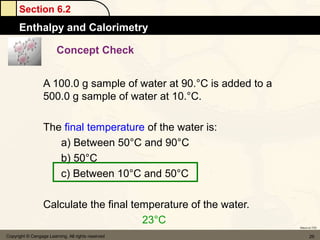
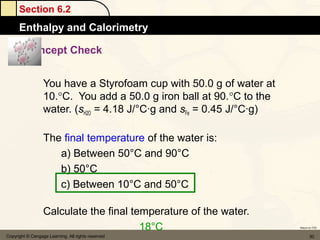







![Section 6.4
Standard Enthalpies of Formation
Conventional Definitions of Standard States
• For a Compound
For a gas, pressure is exactly 1 atm.
For a solution, concentration is exactly
1 M.
Pure substance (liquid or solid)
• For an Element
The form [N2(g), K(s)] in which it exists
at 1 atm and 25°C.
Heat of formation is zero.
Return to TOC
Copyright © Cengage Learning. All rights reserved 42](https://image.slidesharecdn.com/ch-6thermodynamics-121109182115-phpapp02/85/thermodynamics-42-320.jpg)