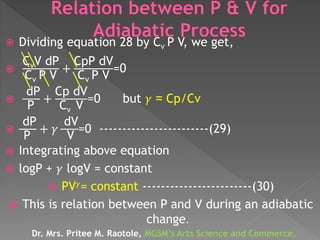
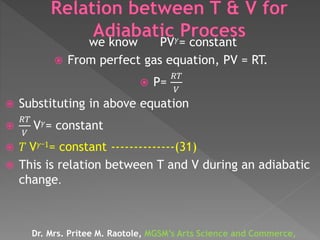
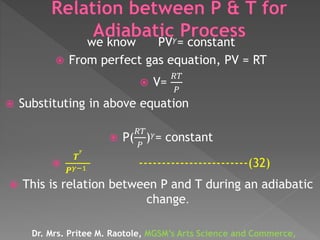
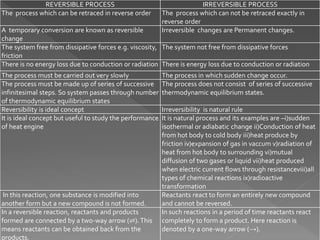
The document discusses concepts related to thermodynamics including:
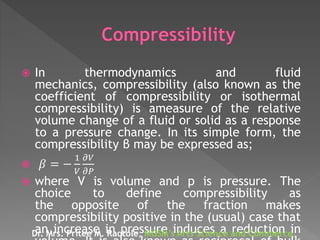
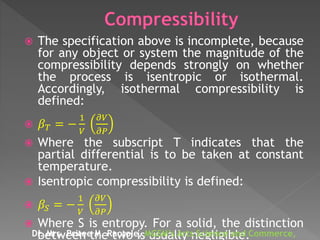
- Compressibility is defined as the relative change in volume of a fluid or solid in response to a pressure change. It can be expressed as the negative partial derivative of volume with respect to pressure.
- Thermal expansion coefficients describe how the size of an object changes with temperature. The volumetric coefficient is the most basic and relevant for fluids. It is defined as the fractional change in size per degree change in temperature at constant pressure.
- Mathematical definitions are provided for volumetric, area, and linear coefficients of thermal expansion in solids, liquids, and gases. The volumetric coefficient is the partial derivative of volume with respect to temperature at