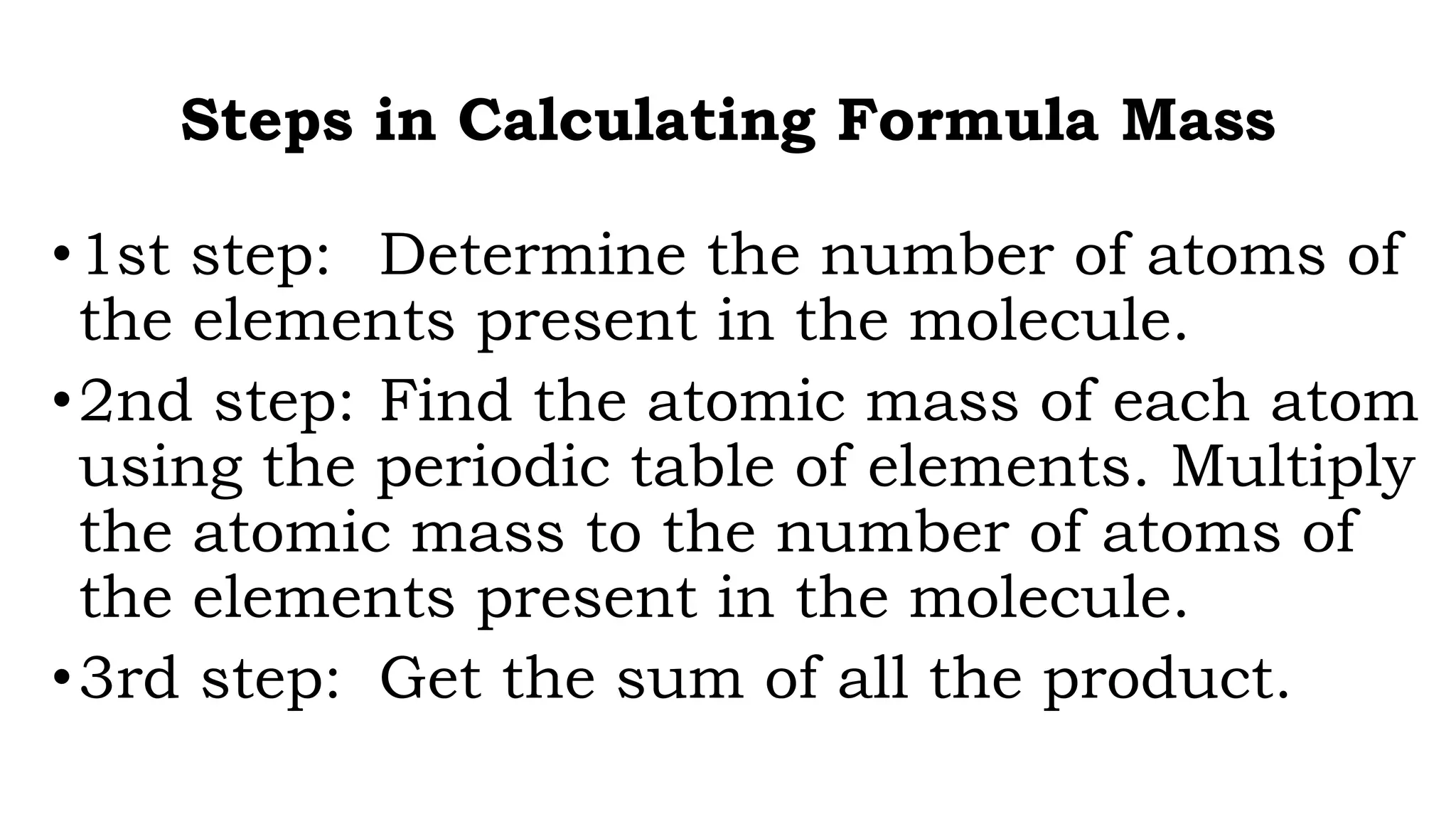
The document discusses the mole concept and how to calculate molecular and formula mass. It defines formula mass as the sum of the atomic weights in an empirical formula, and molecular mass as the sum from the molecular formula. It provides steps to calculate molecular mass by determining atoms, finding atomic masses from the periodic table, and summing the products. The document also defines a mole as a quantity containing 6.02x1023 particles, the number of atoms in 12g of carbon-12. It can be used to convert between mass and number of moles.