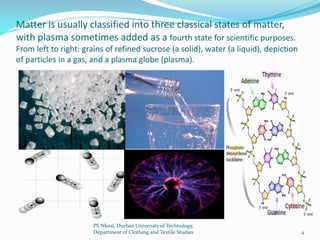
This document contains the syllabus for a chemistry course at Durban University of Technology. The syllabus covers the following topics over one semester: basic concepts about matter including states of matter, physical and chemical properties; atomic structure and the periodic table; chemical bonding and intermolecular forces; the mole concept and balancing chemical equations; solutions; acids and bases; oxidation and reduction; and introductions to organic and inorganic chemistry. The document provides definitions and examples for key concepts like elements, compounds, mixtures, molecules, and atoms. It also outlines physical and chemical properties of metals and non-metals.