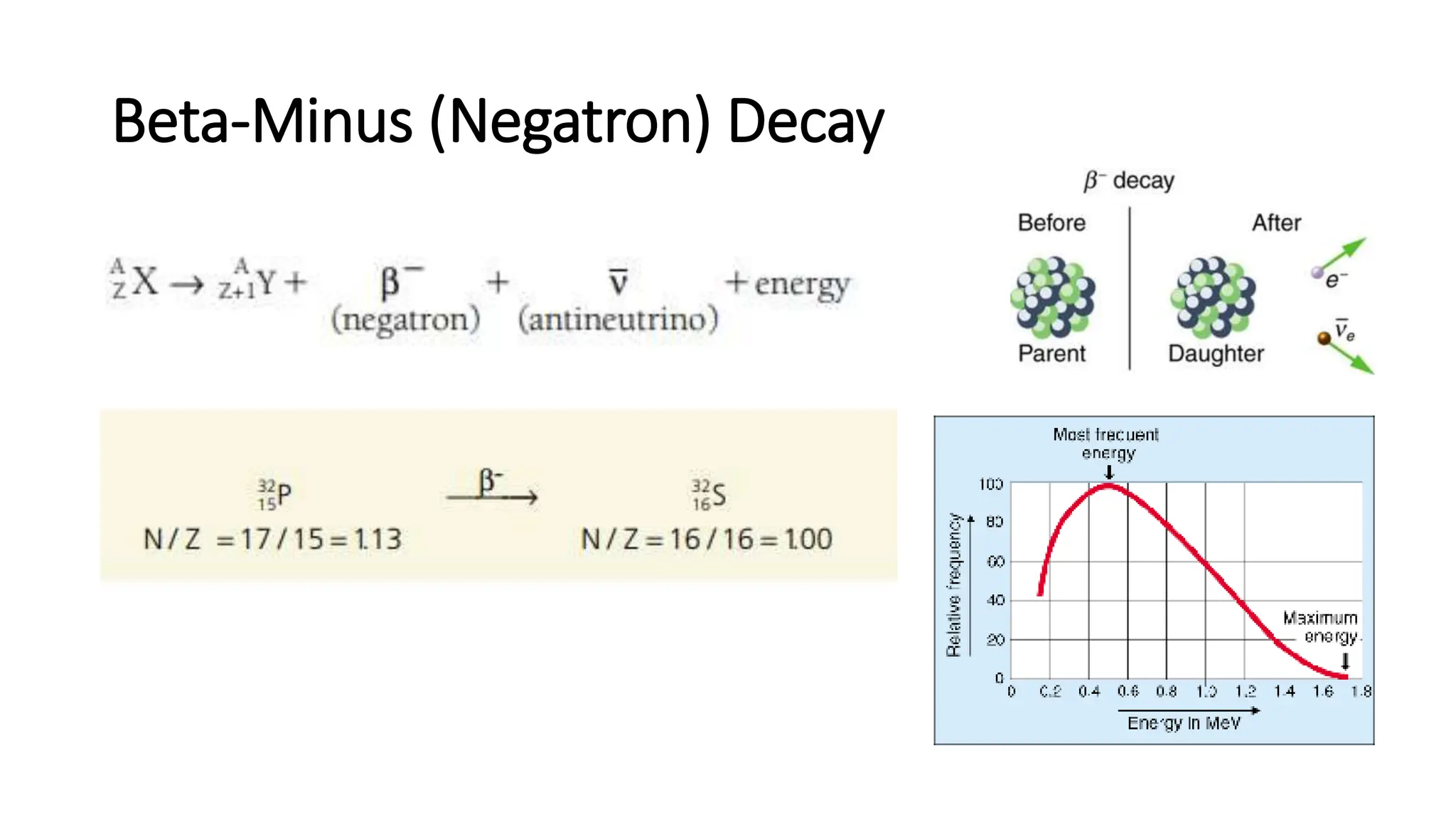
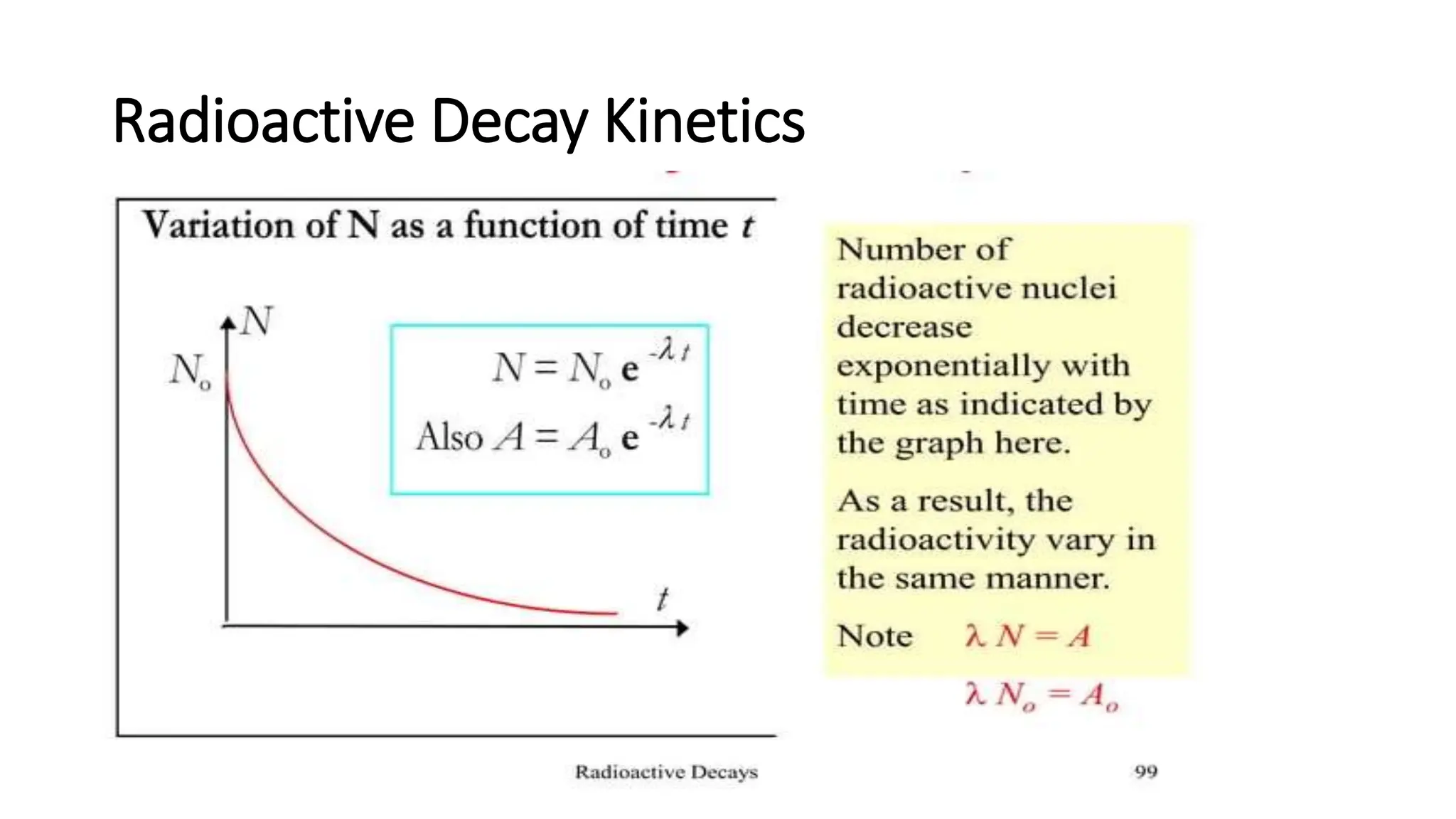
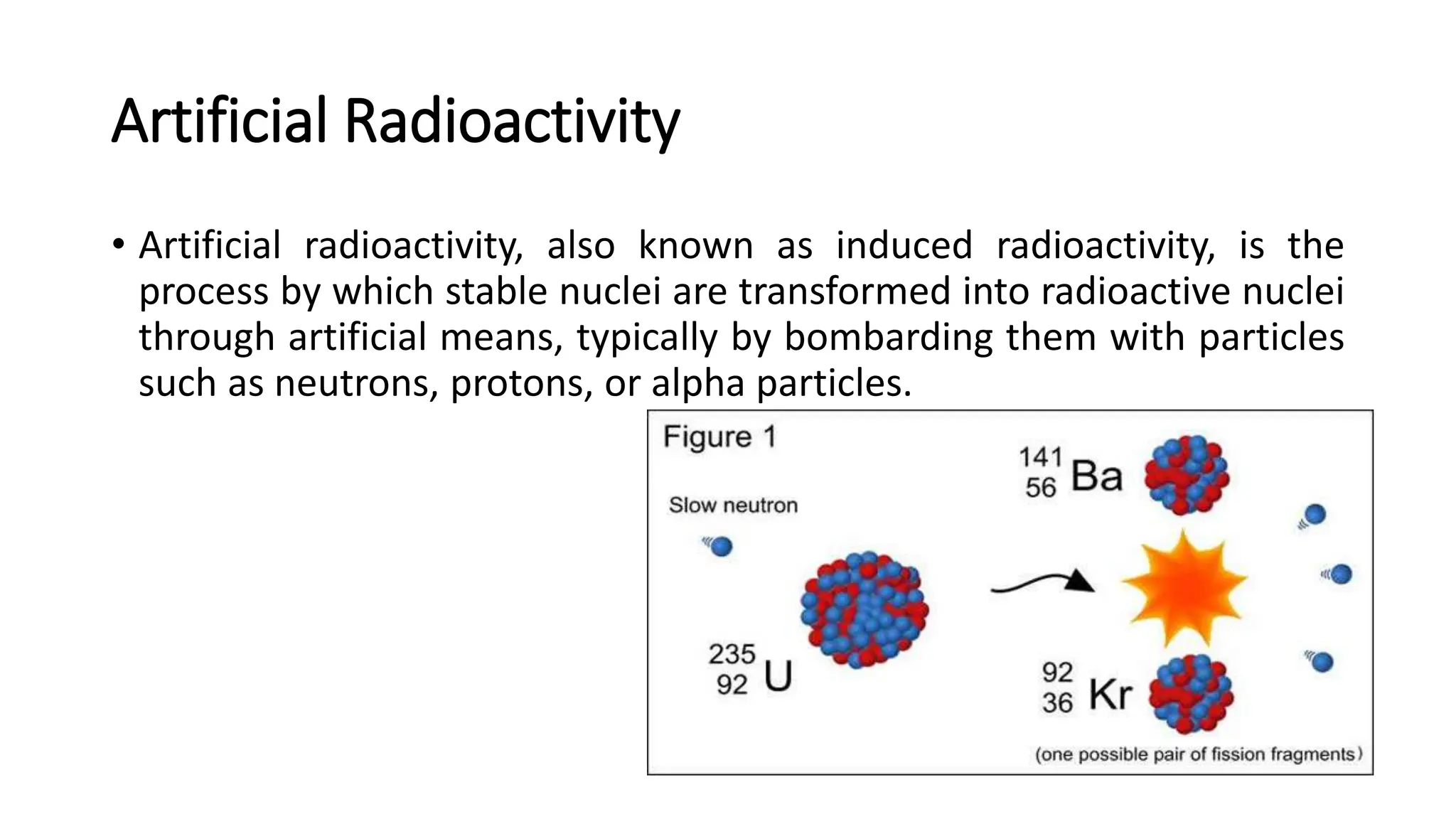
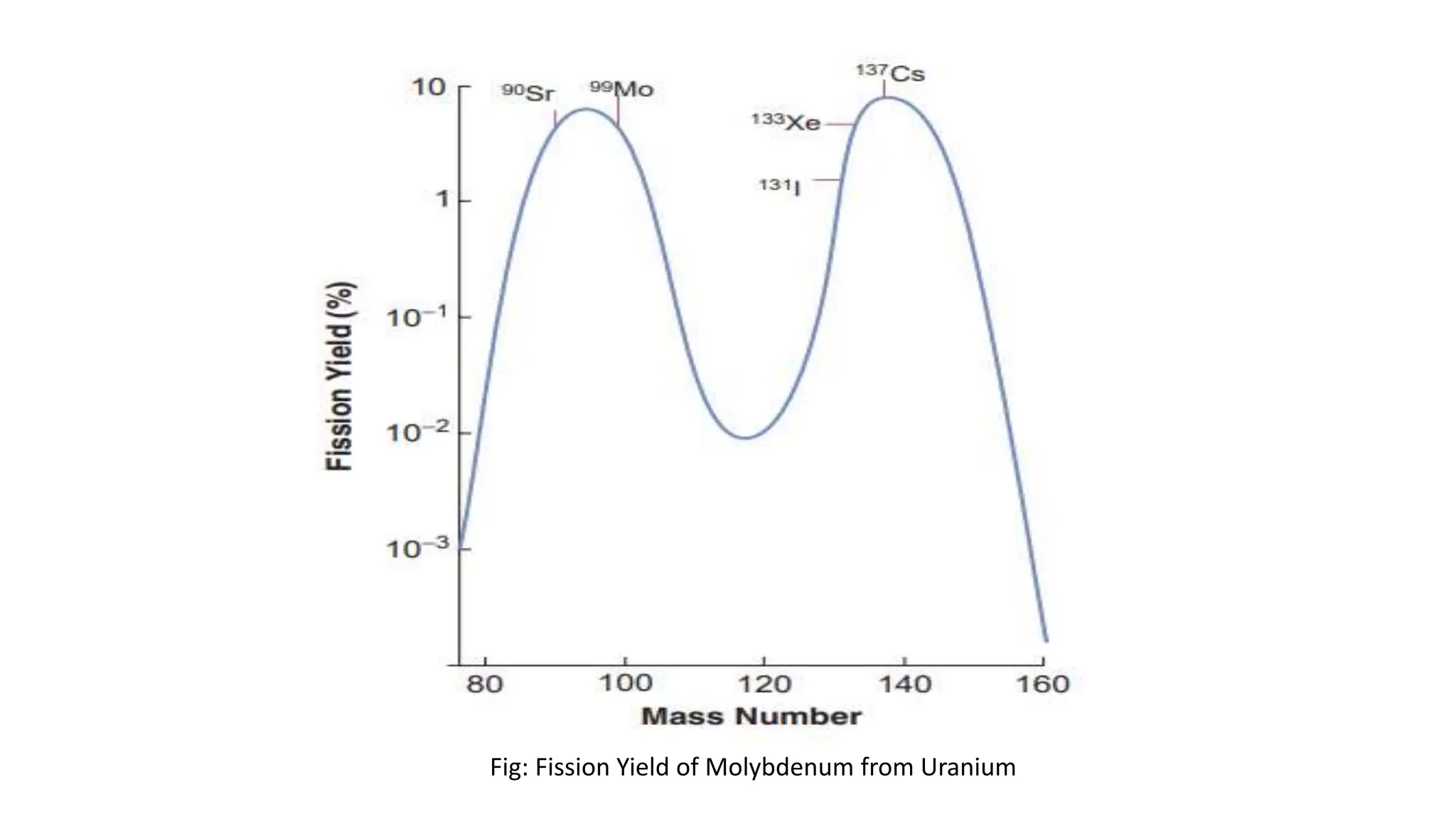
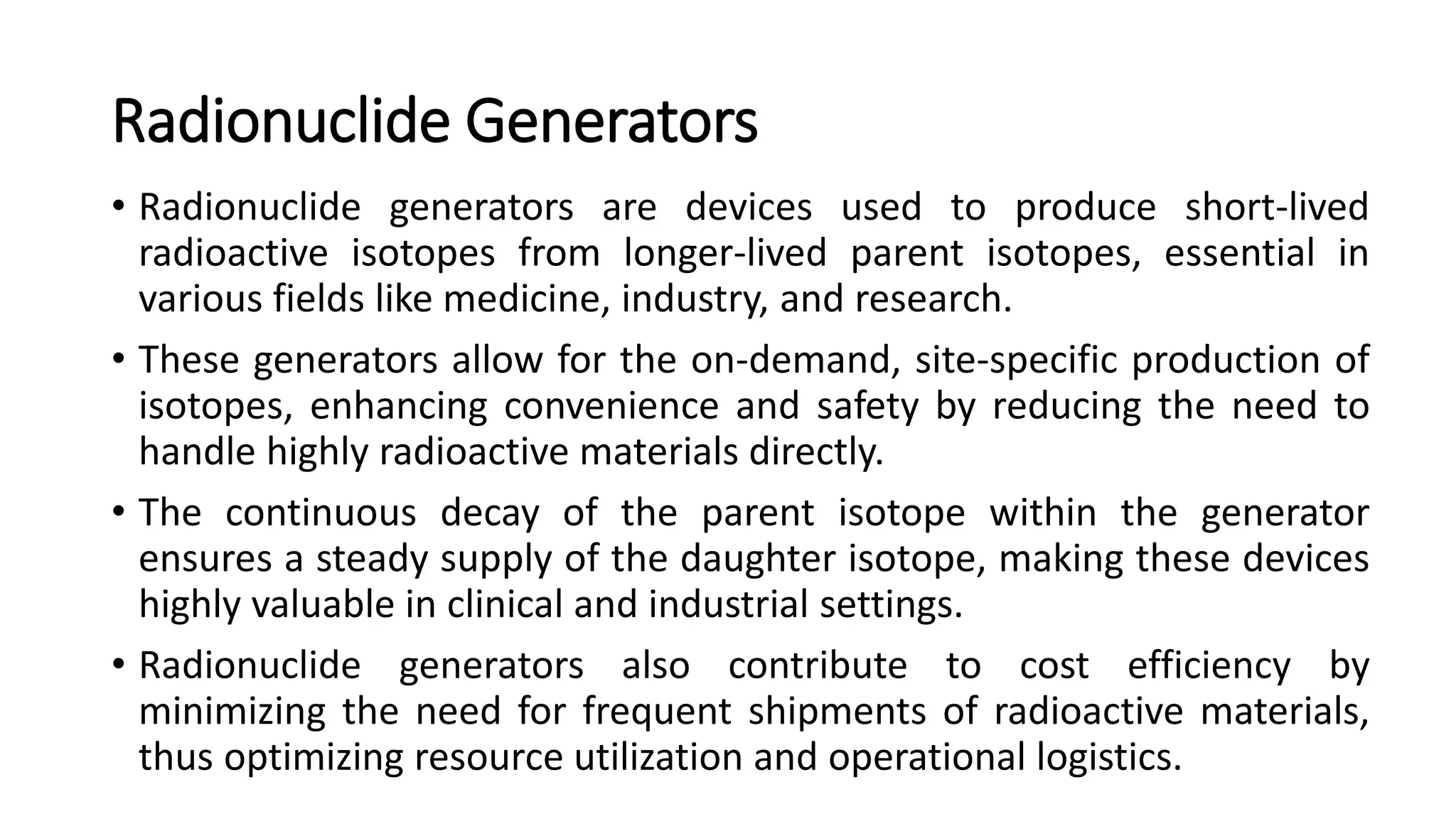
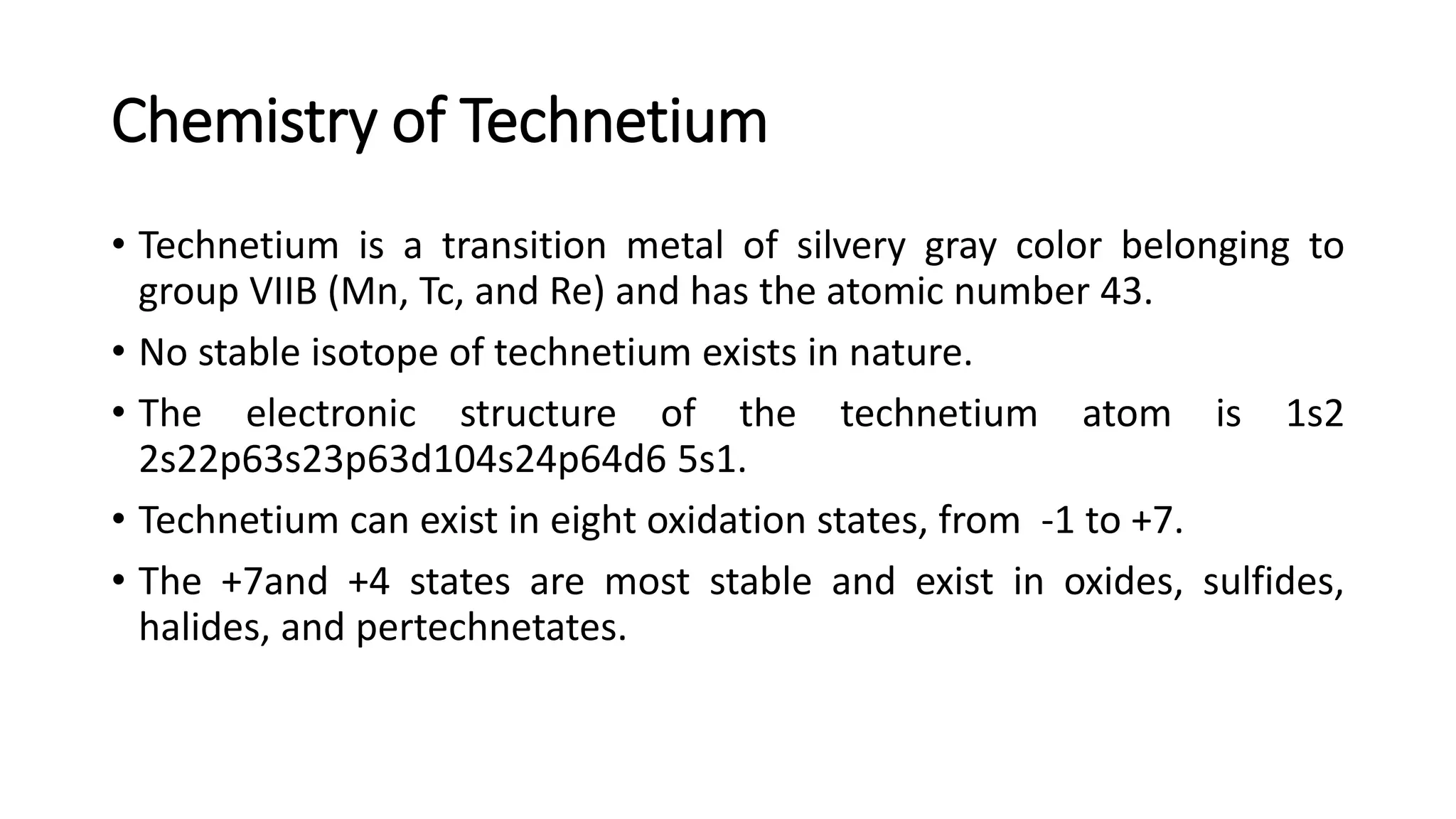
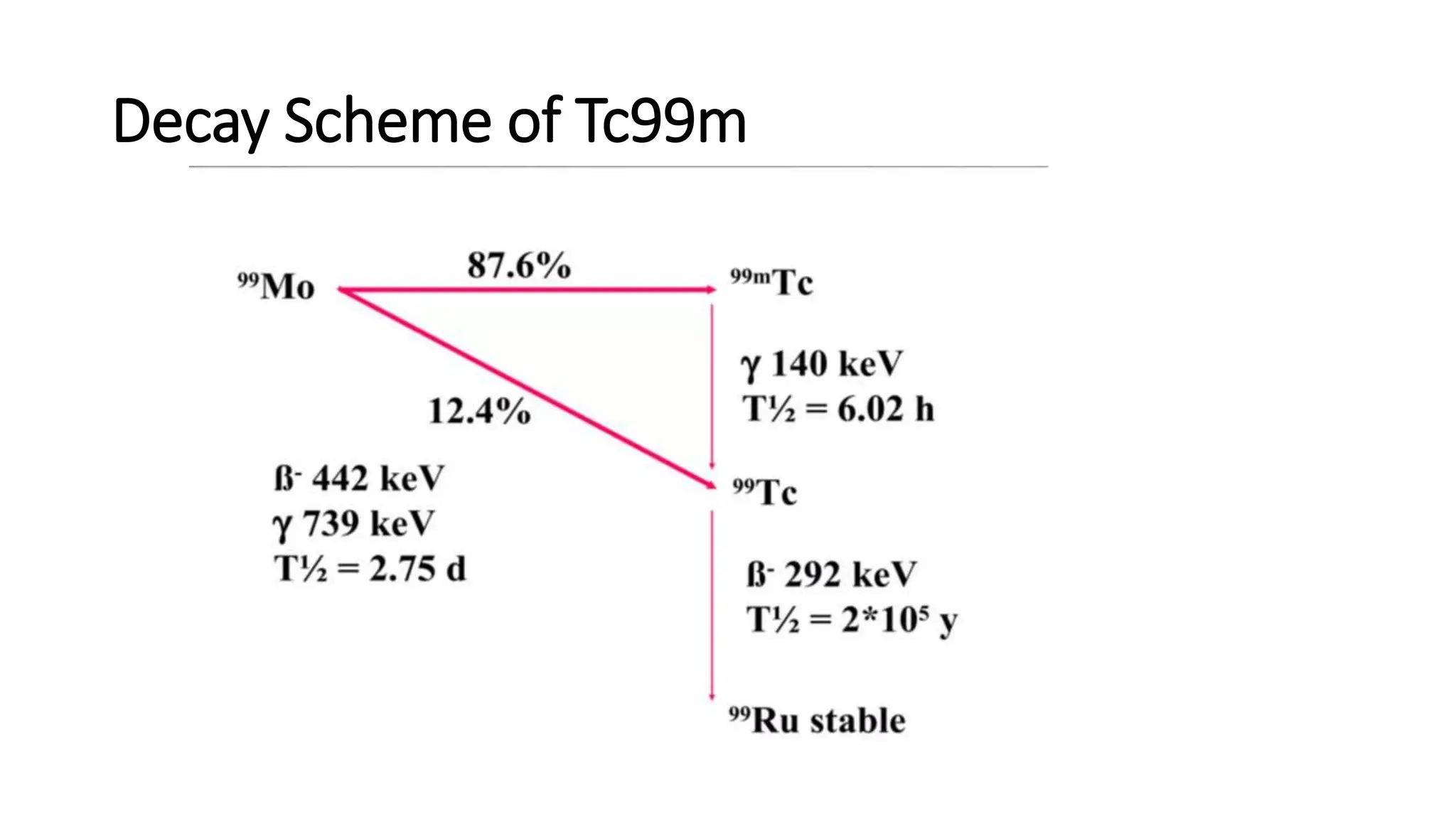
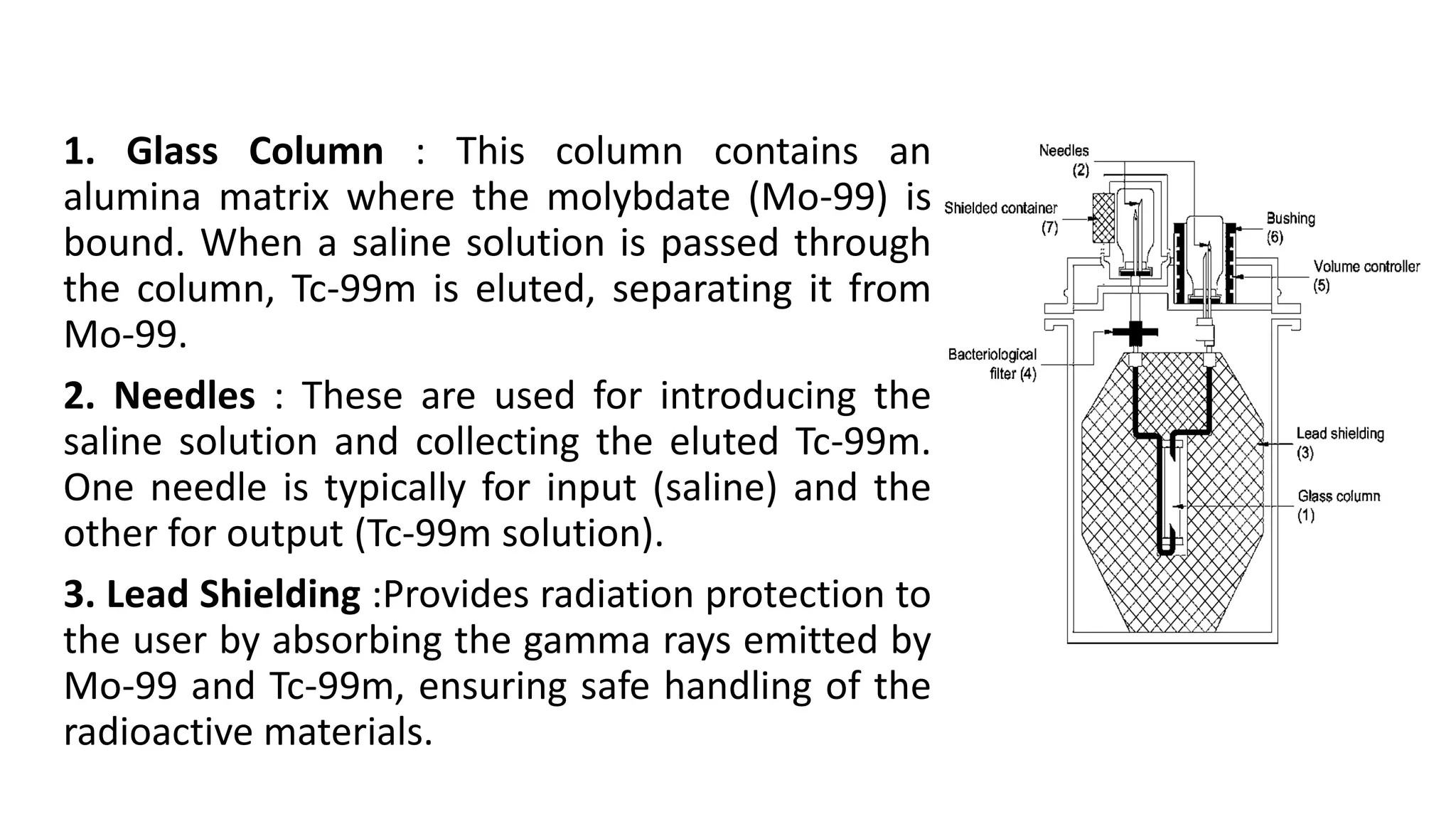
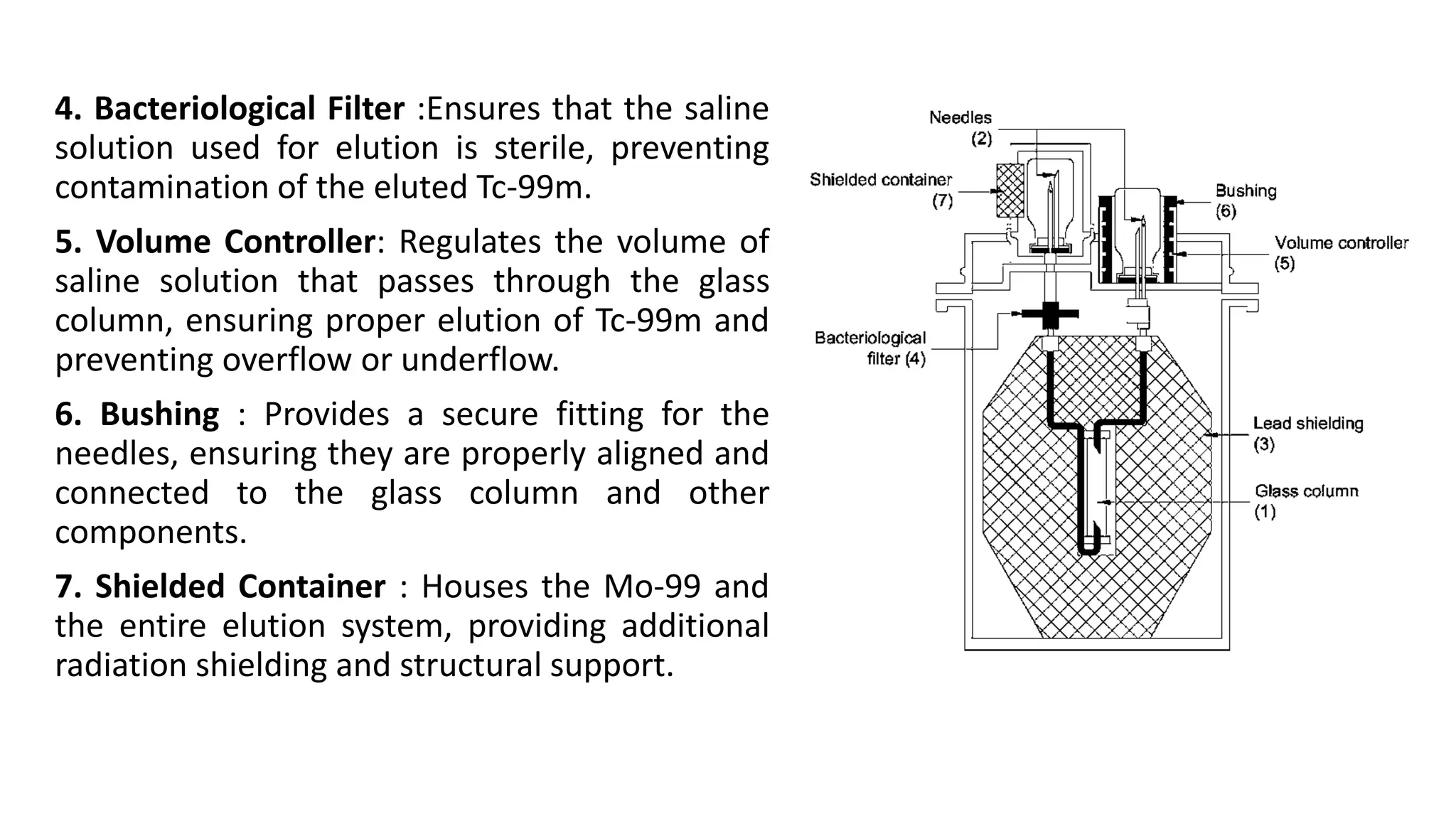
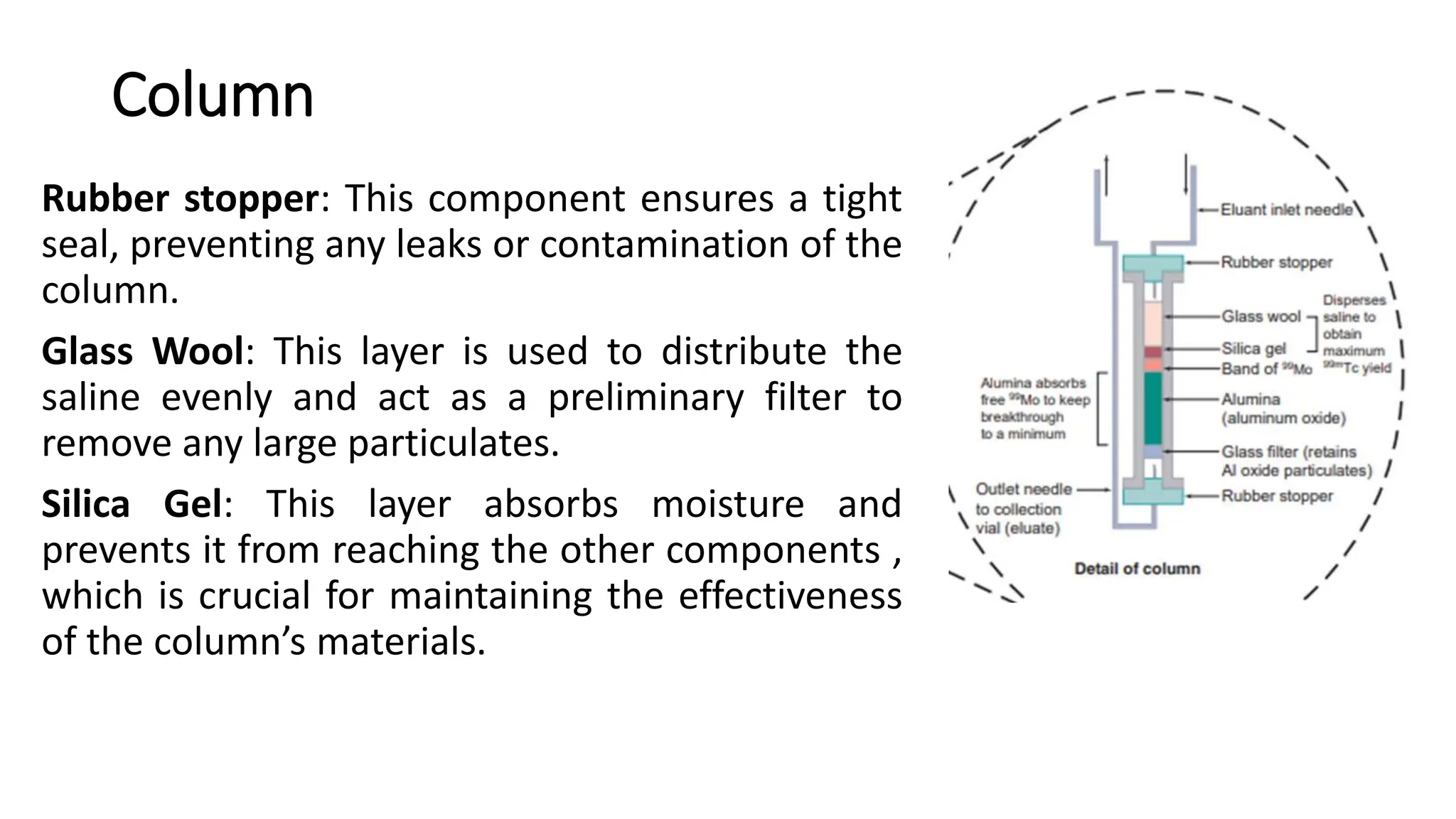
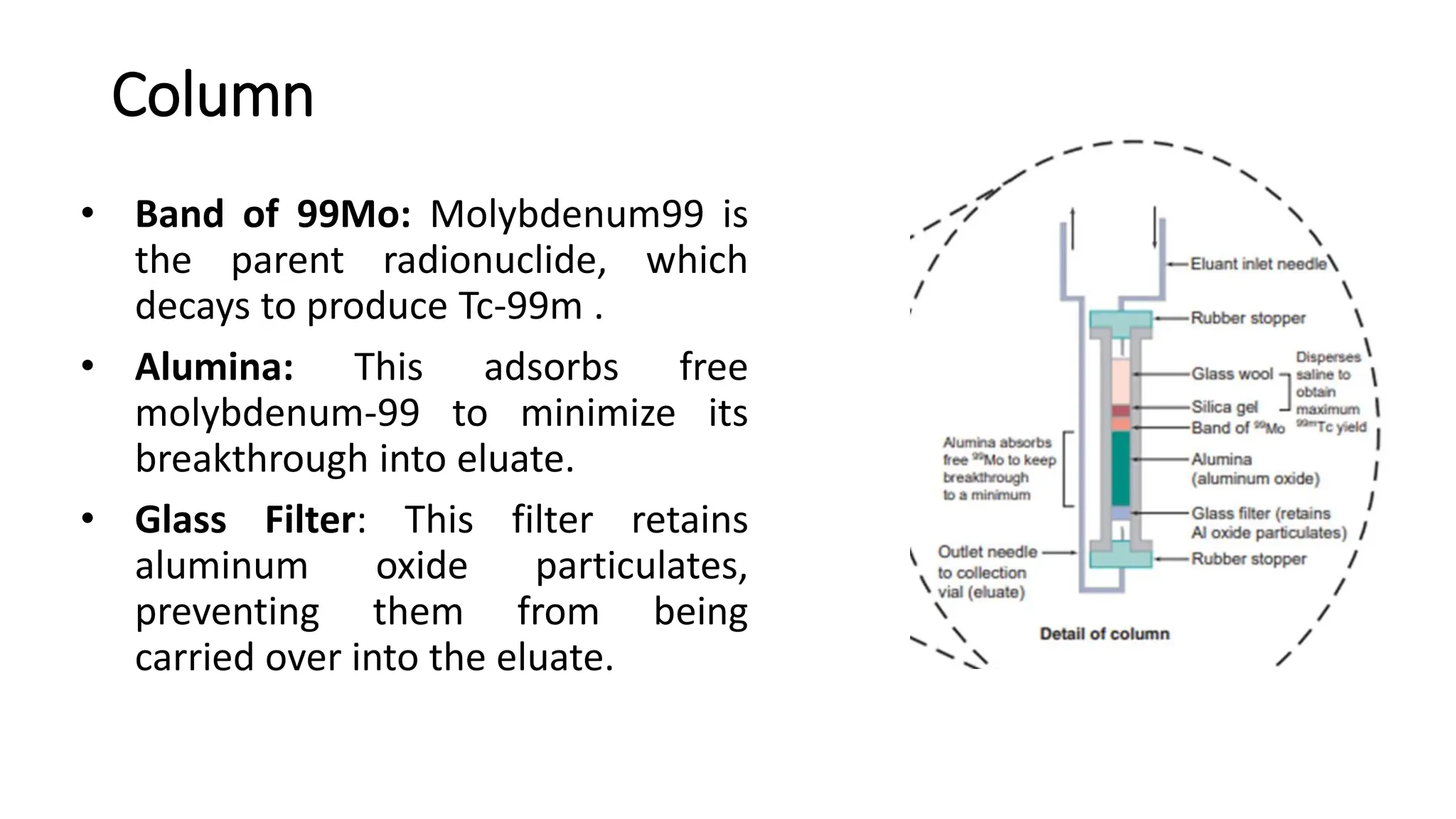
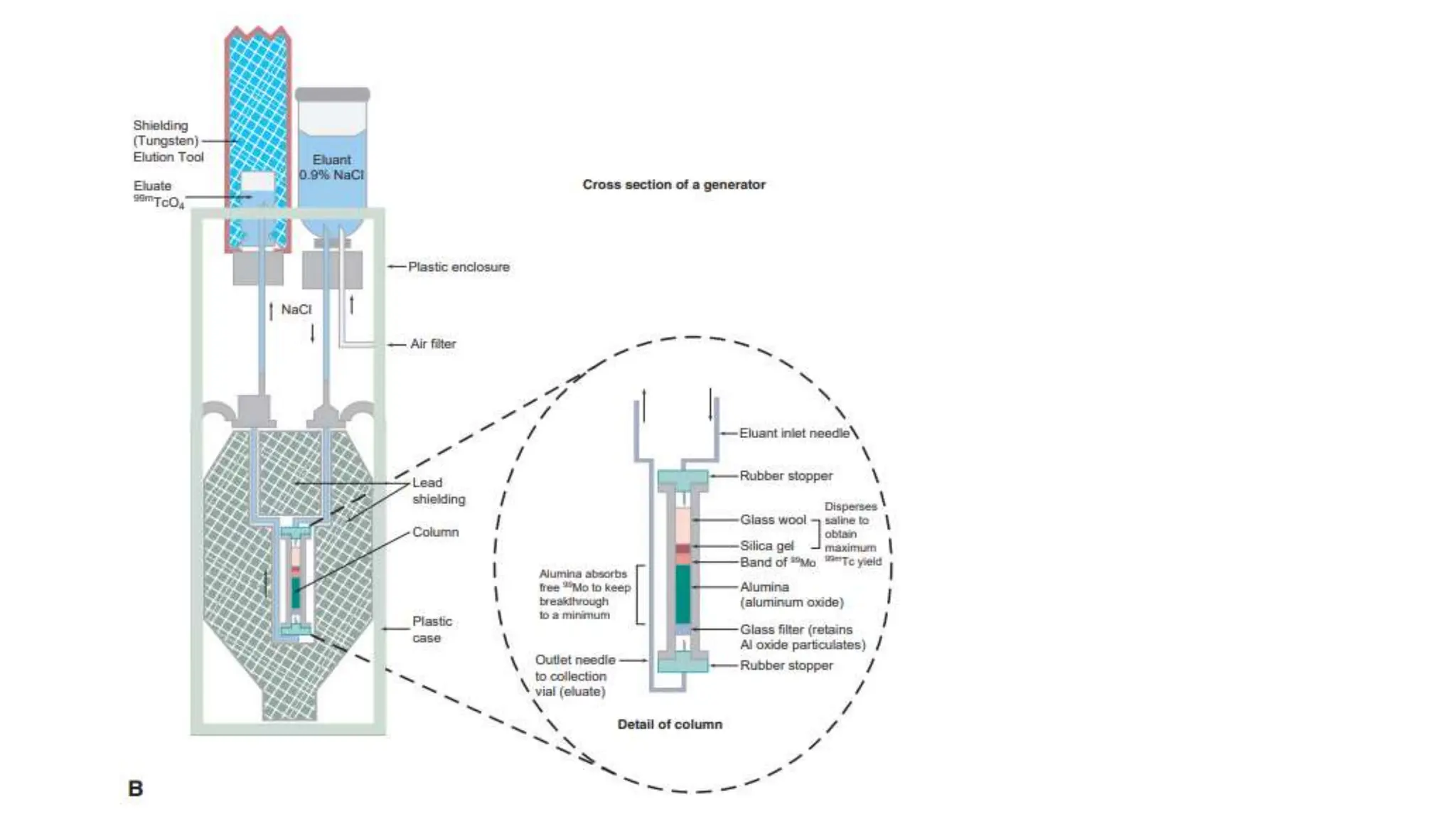
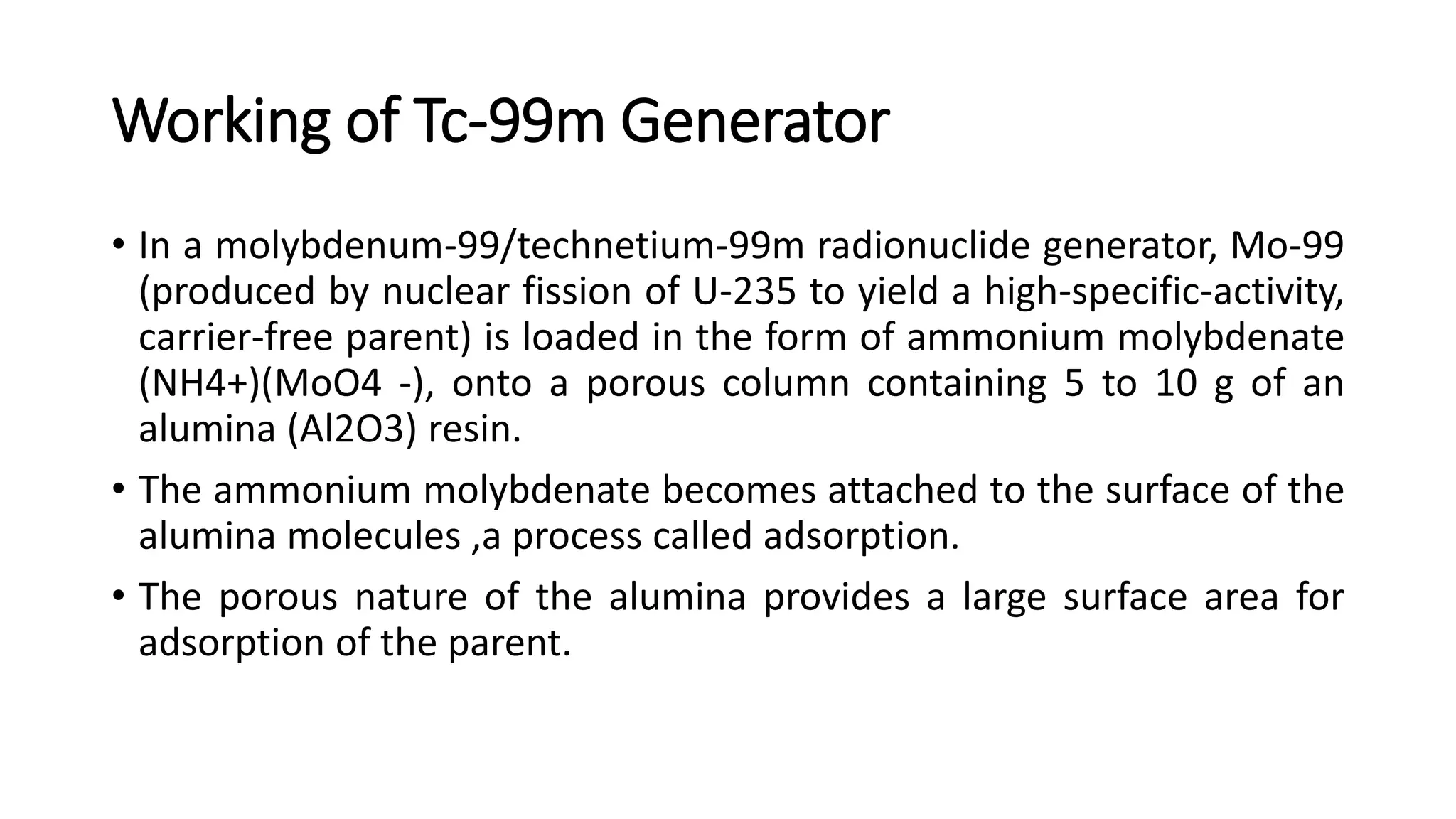
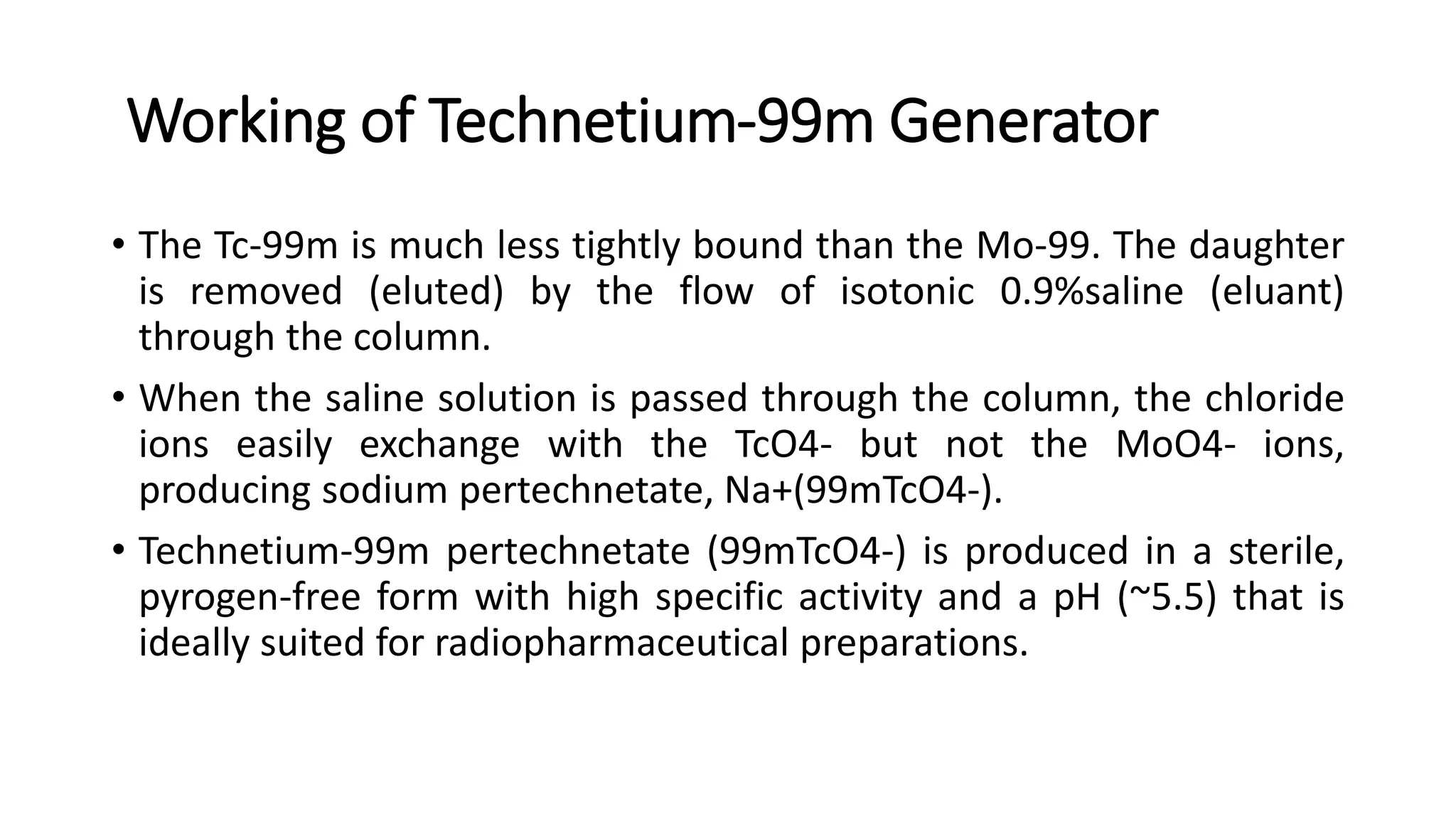
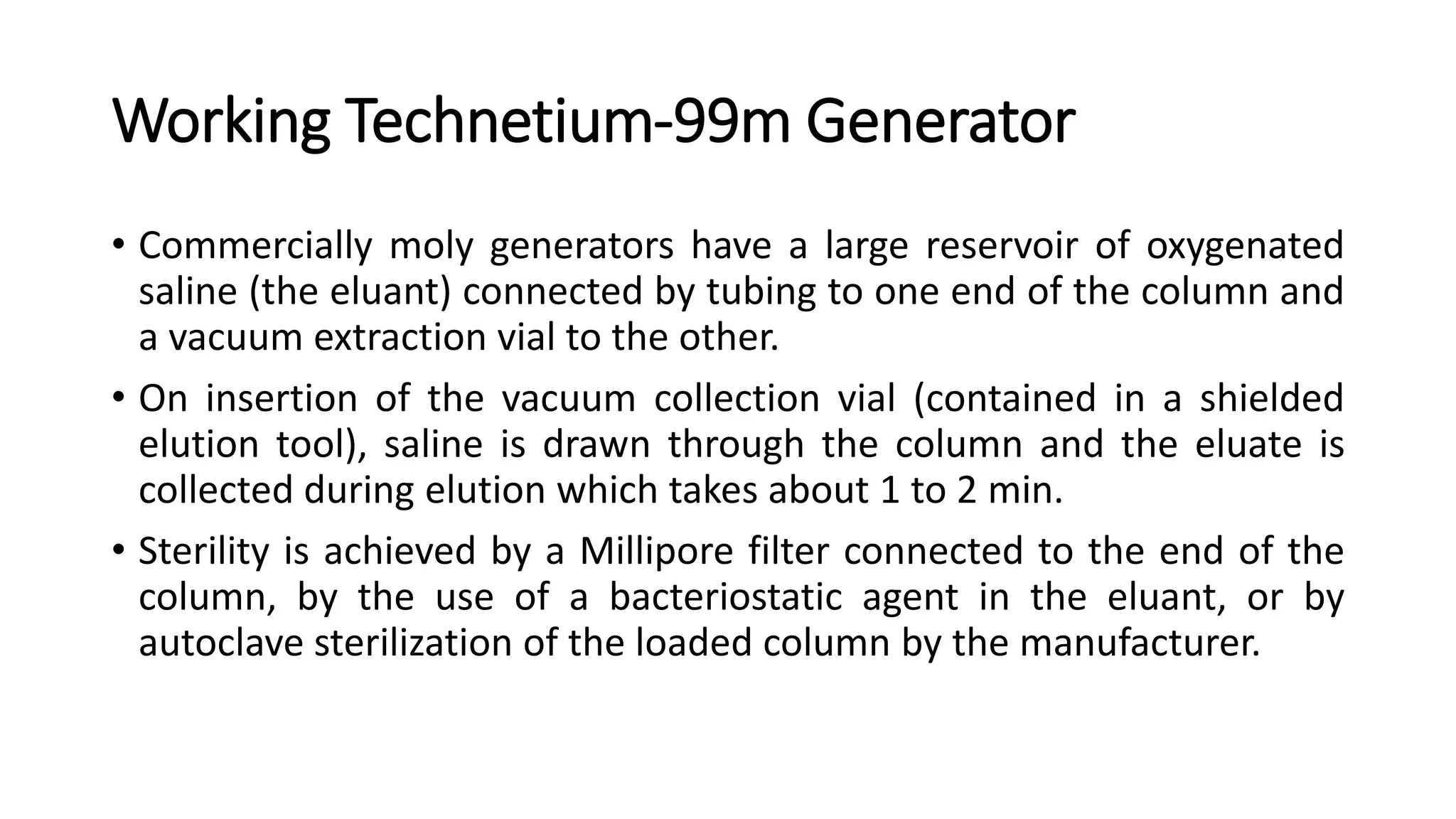
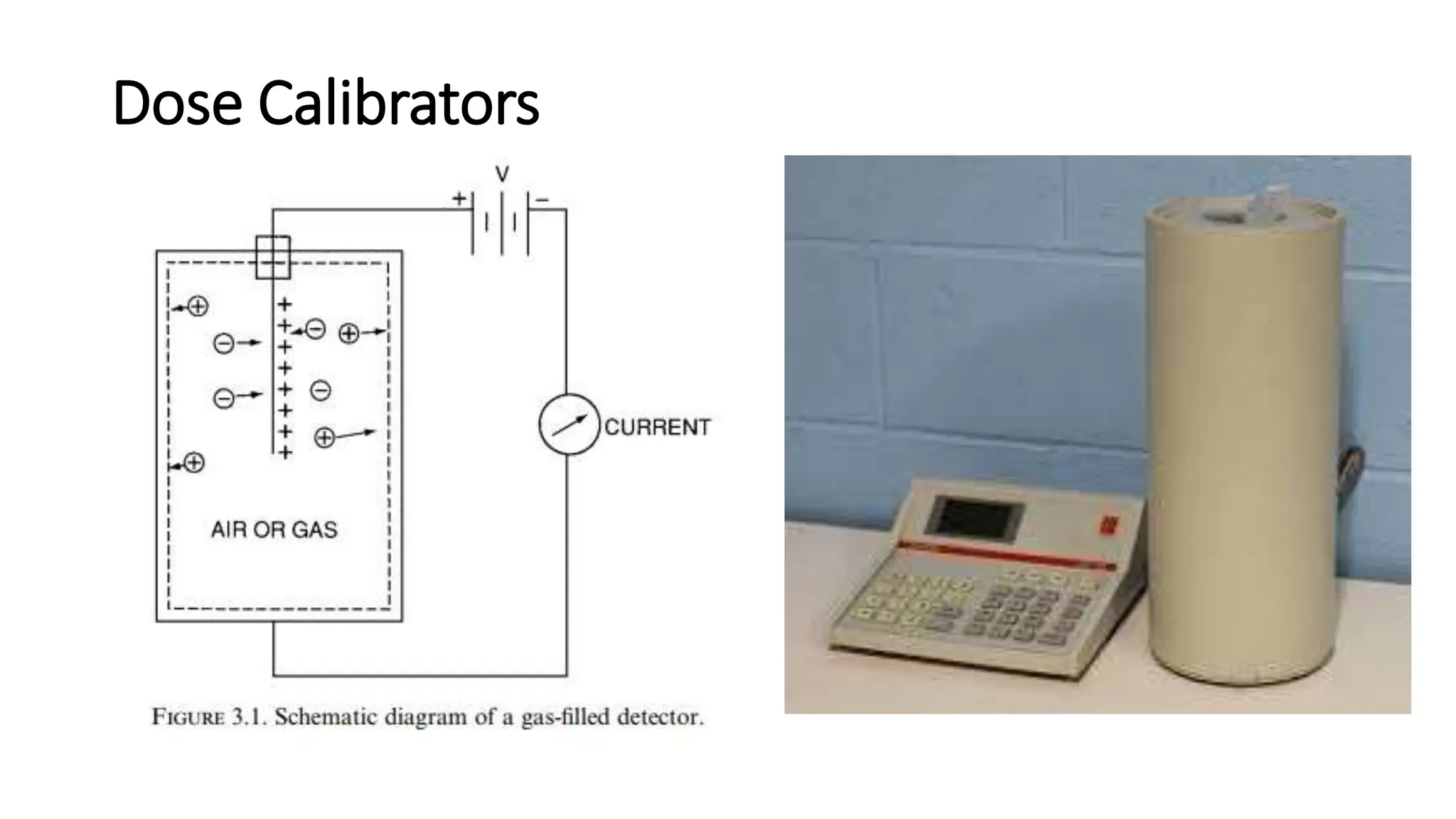
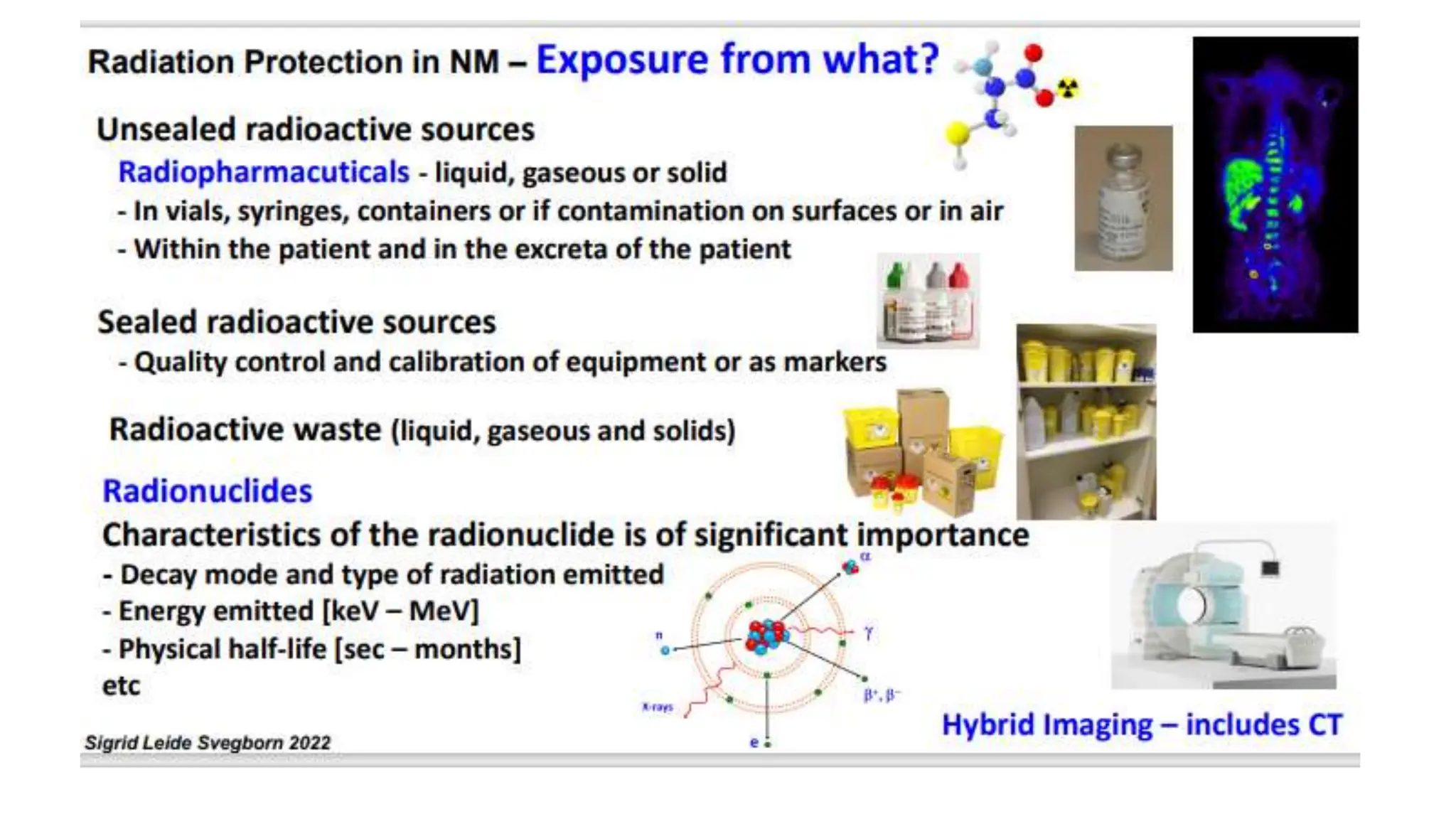
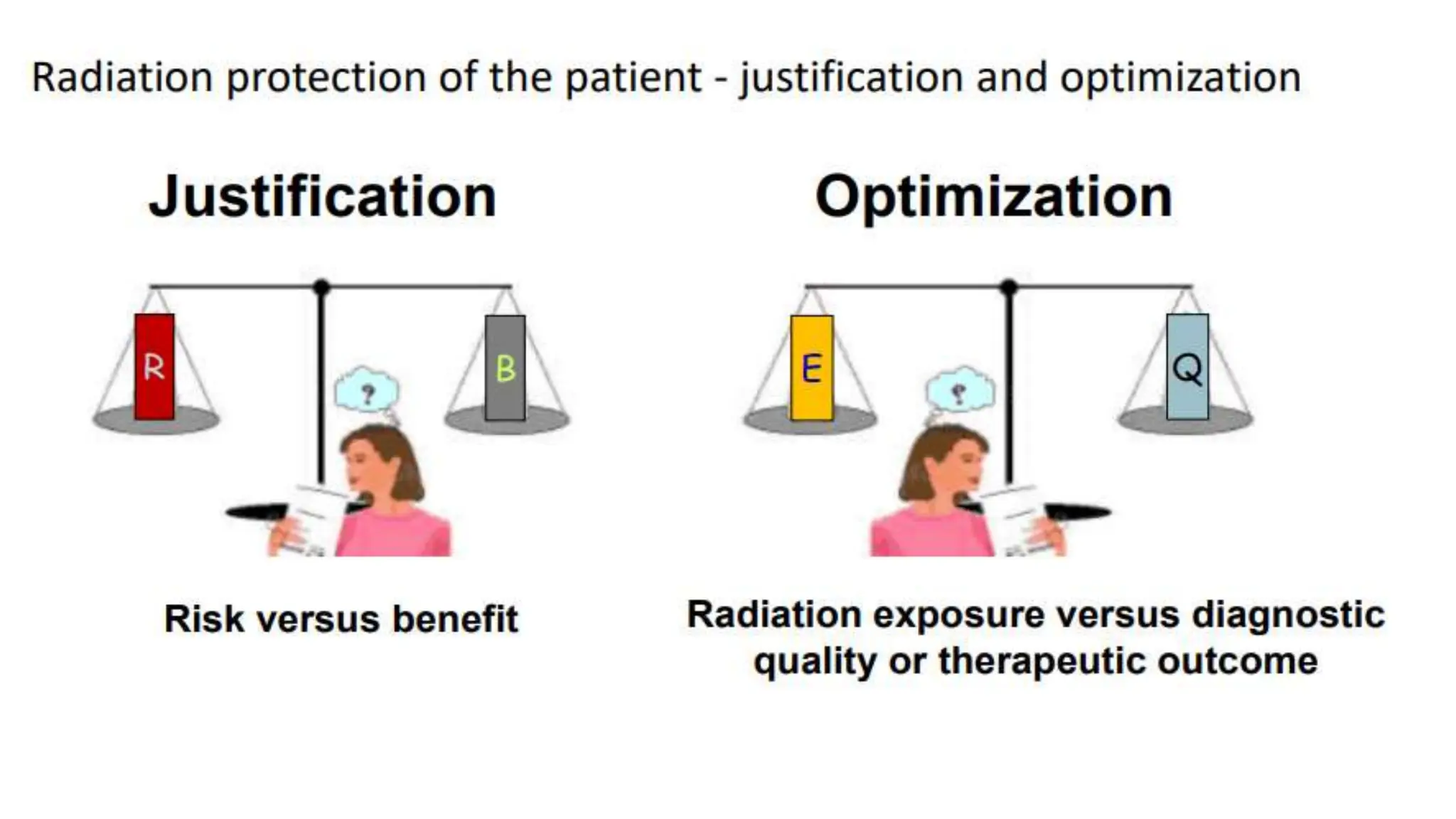
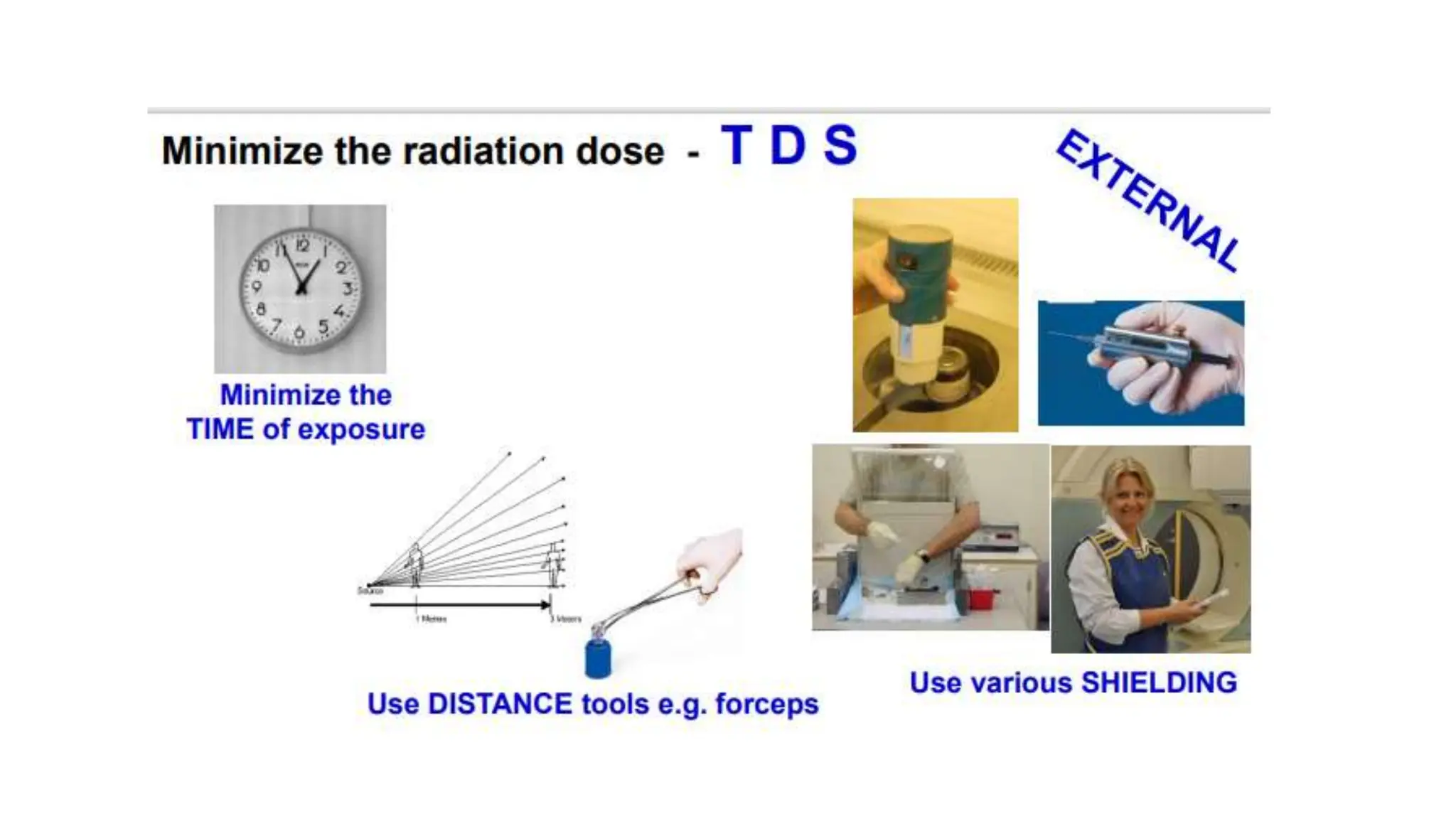
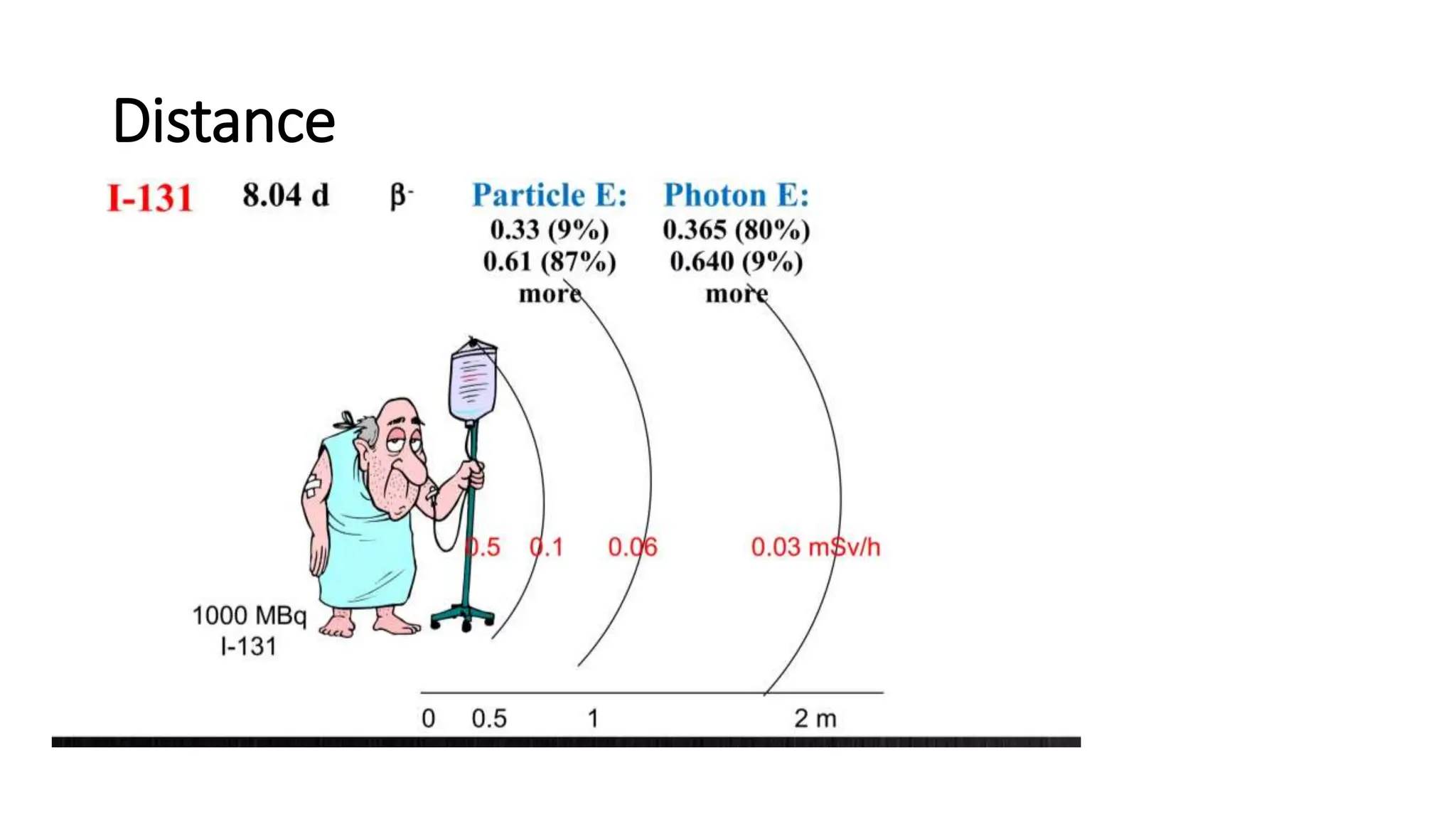
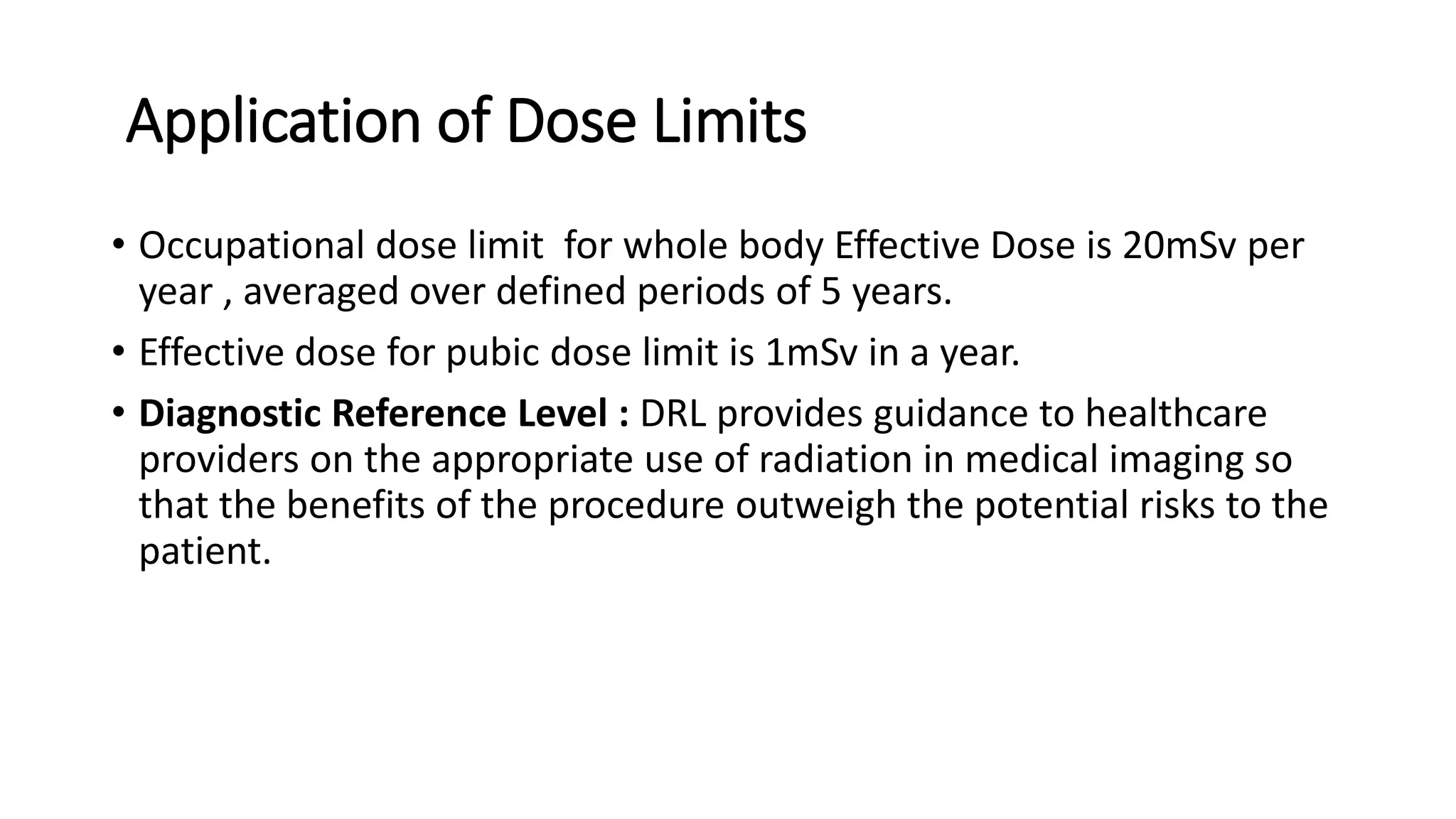
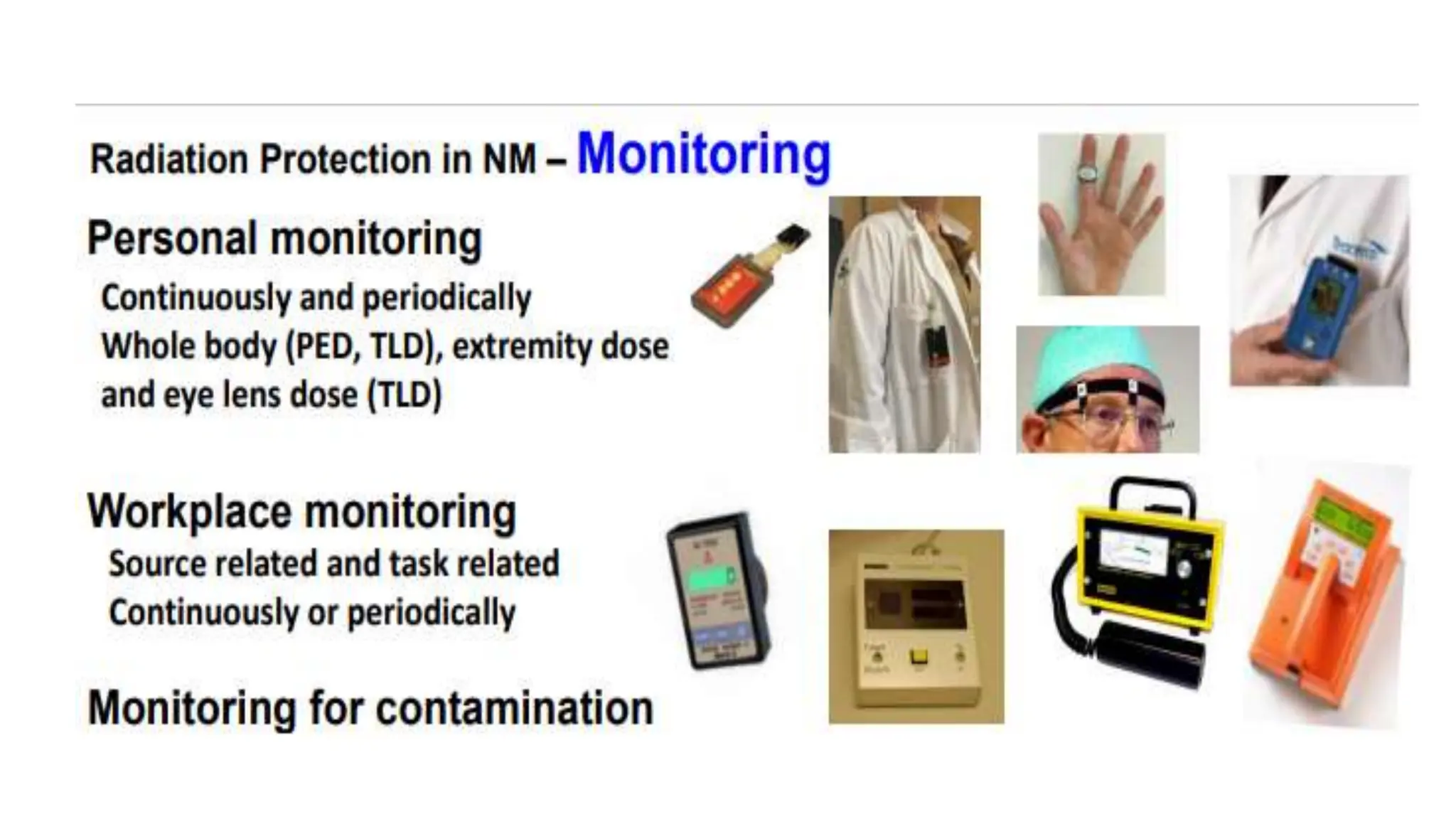
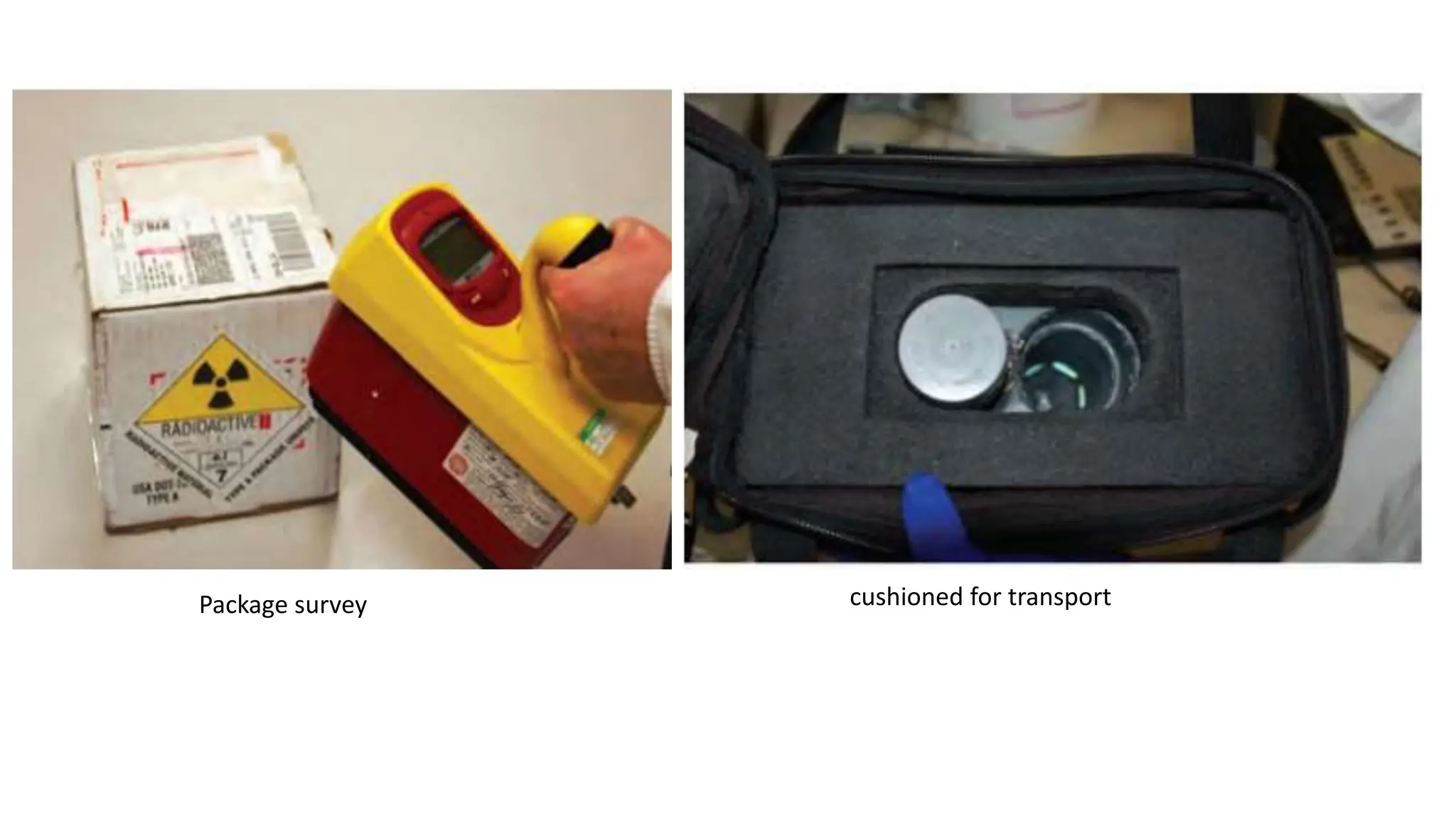
The document outlines the principles and practices of using technetium-99m generators for producing radiopharmaceuticals, emphasizing the processes of radioactivity, the importance of radionuclide generators in medicine, and the safety measures required when handling radioactive materials. Key topics include artificial radioactivity, the chemistry and decay of technetium, the design and operation of technetium-99m generators, and the regulations for safe use and exposure limits in a clinical setting. Additionally, it addresses contamination issues, safe laboratory practices, and the procedure for managing spills and accidents.