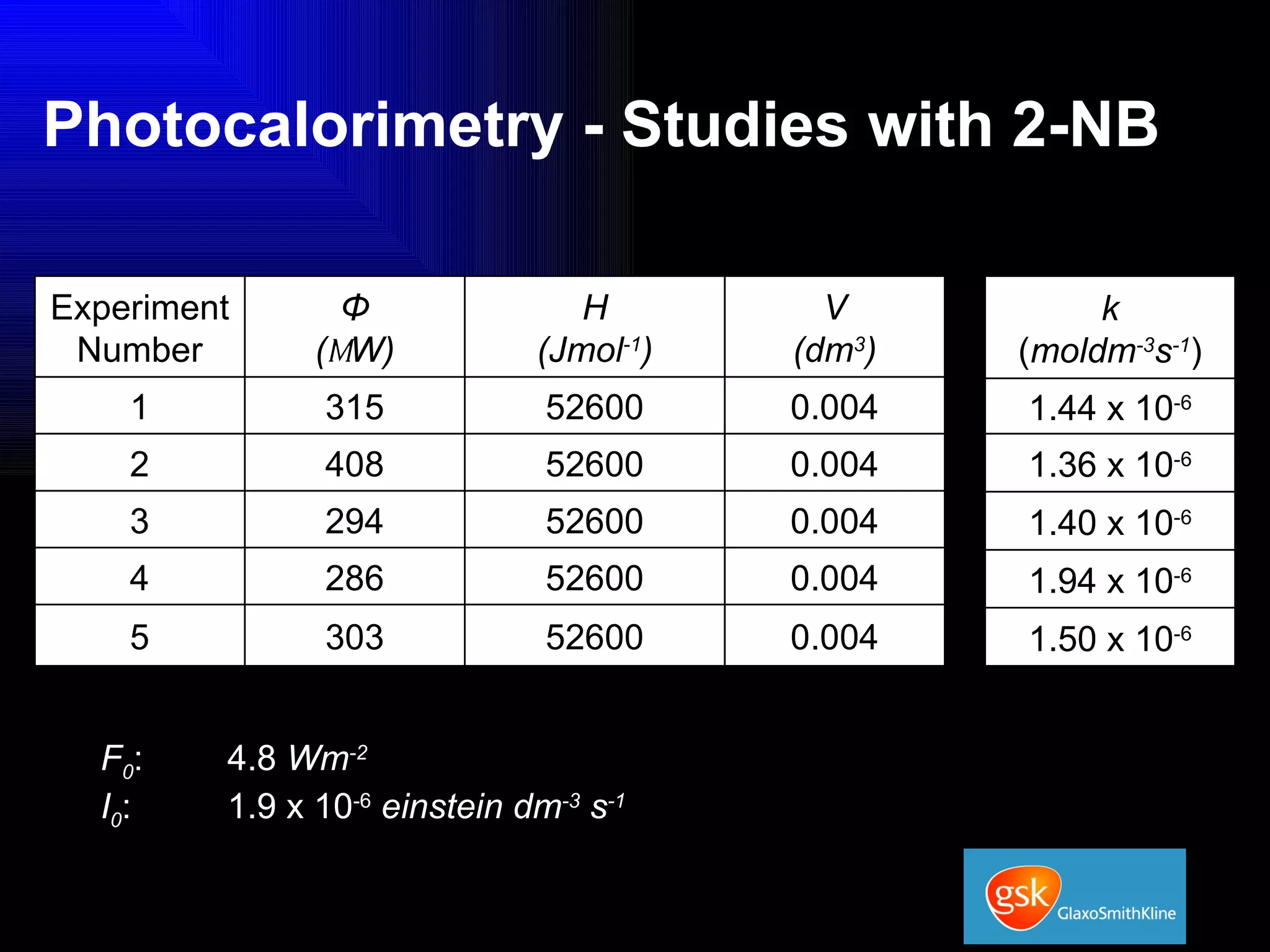
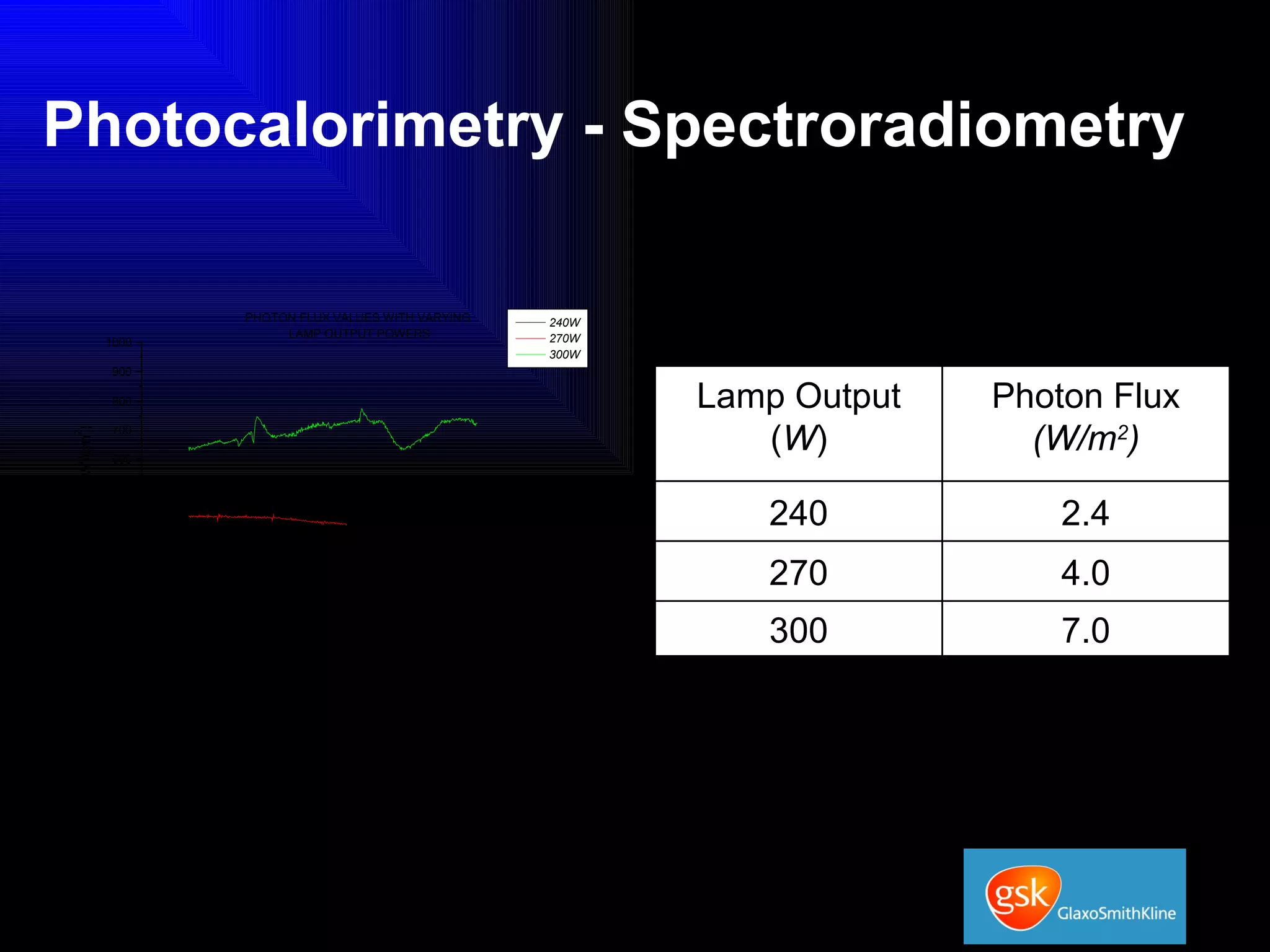
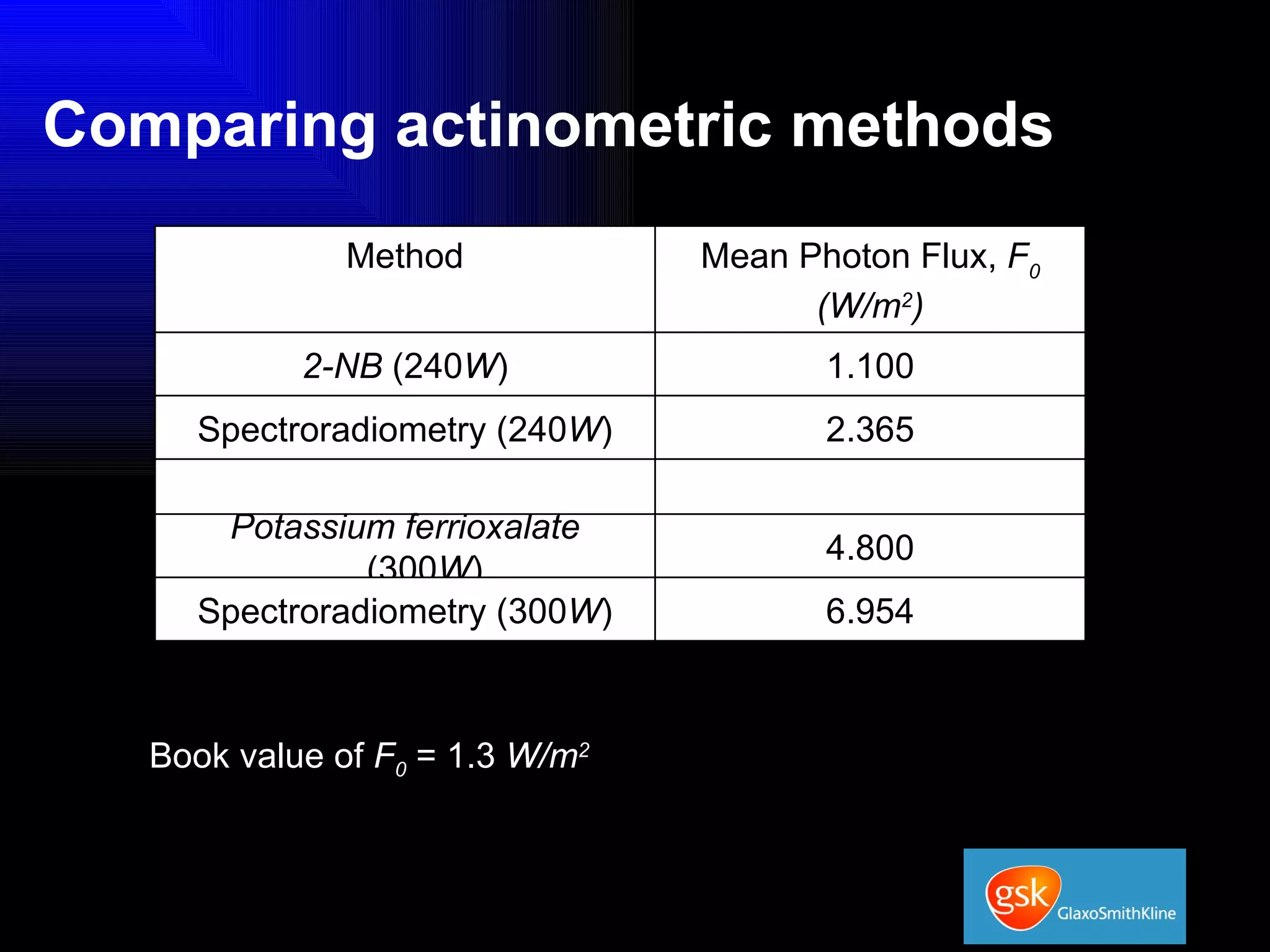
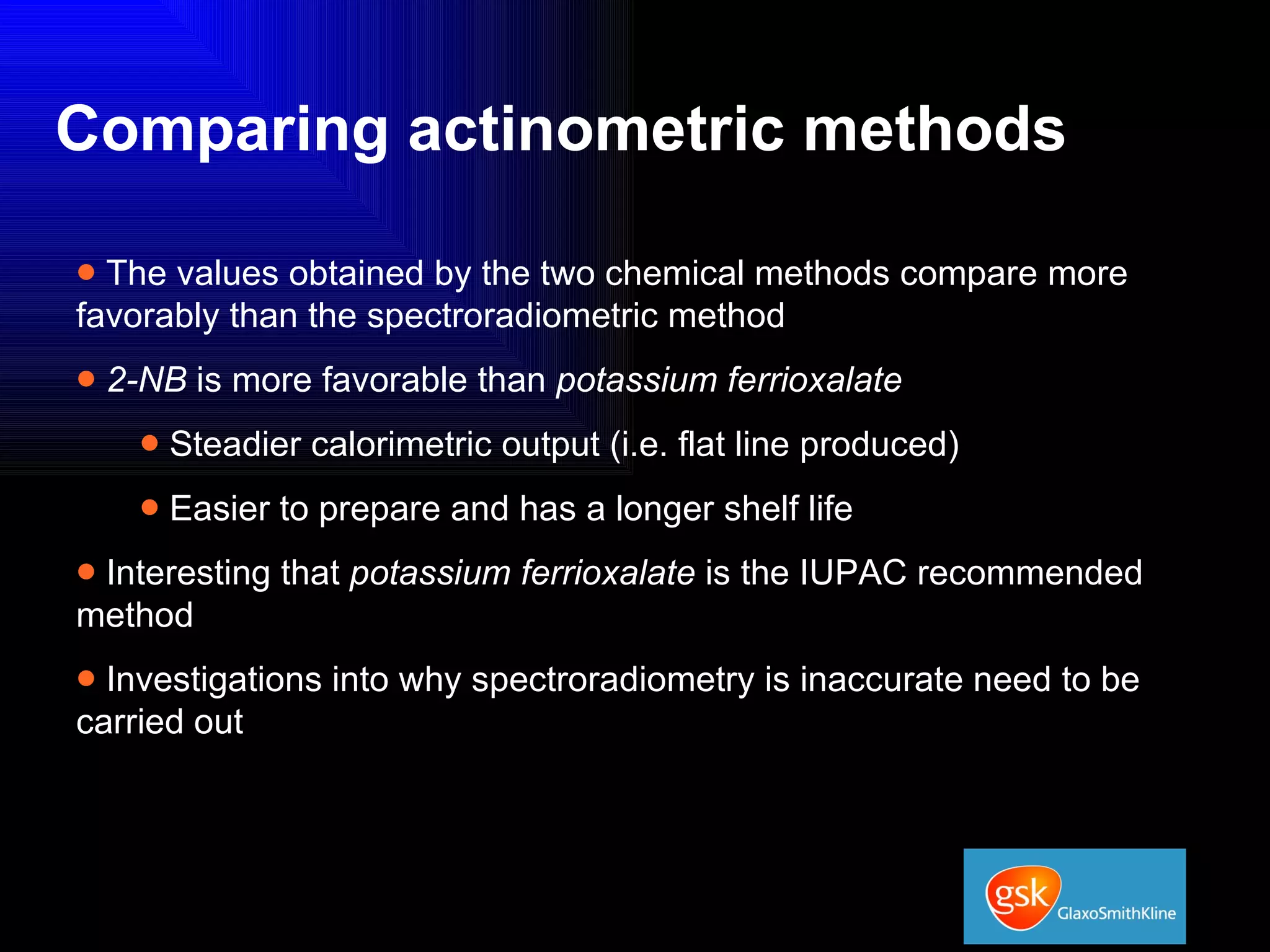
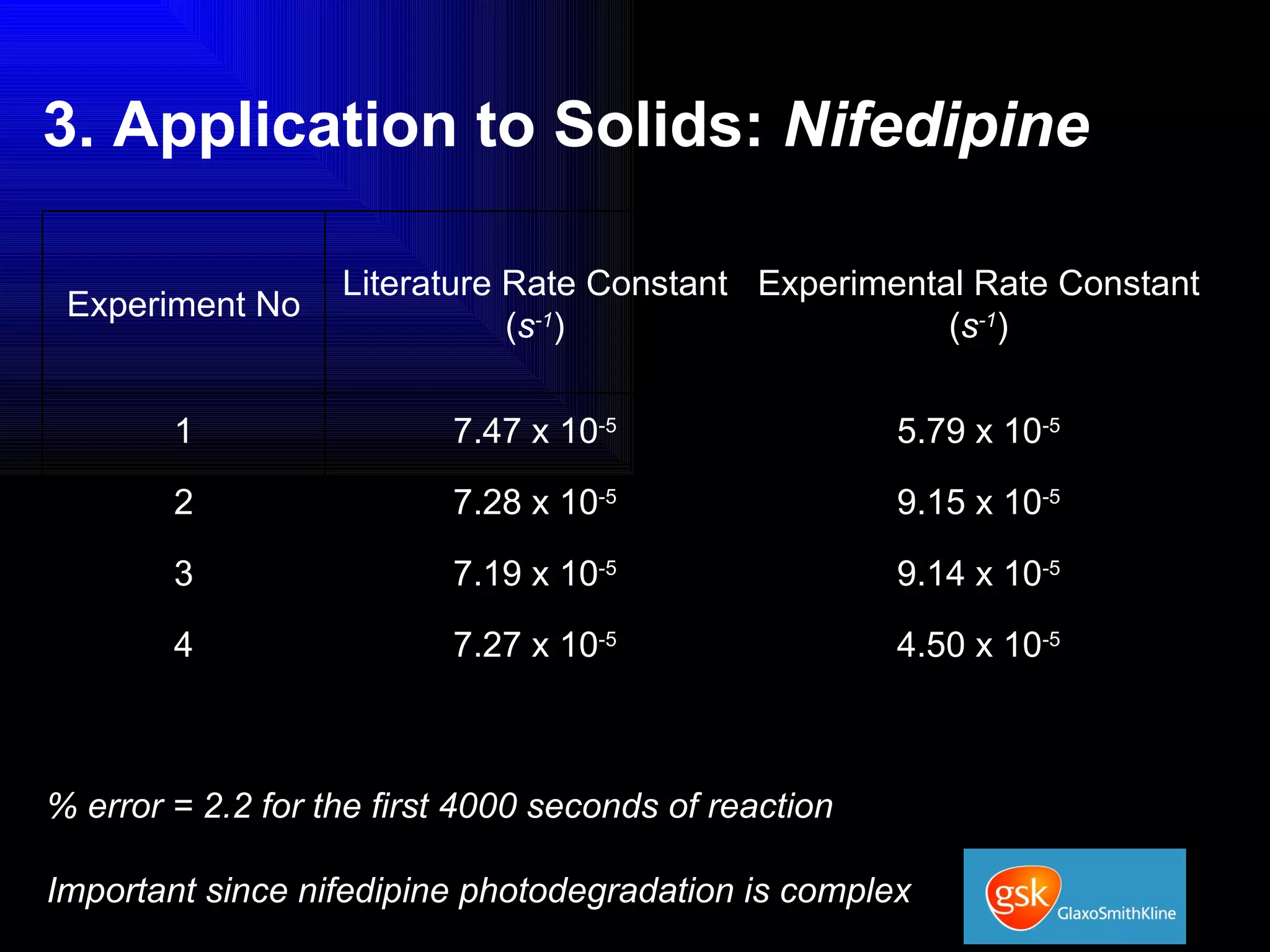
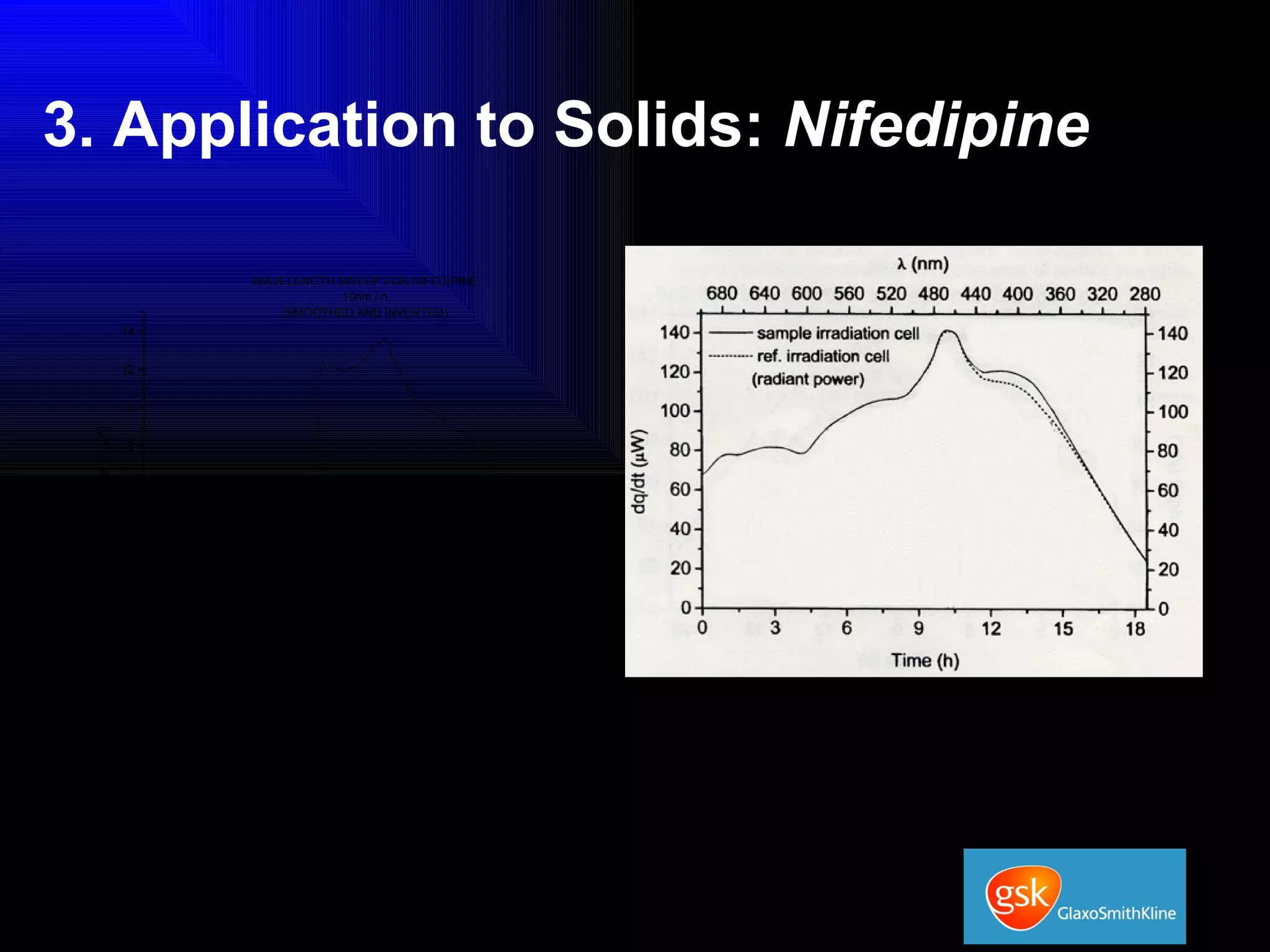
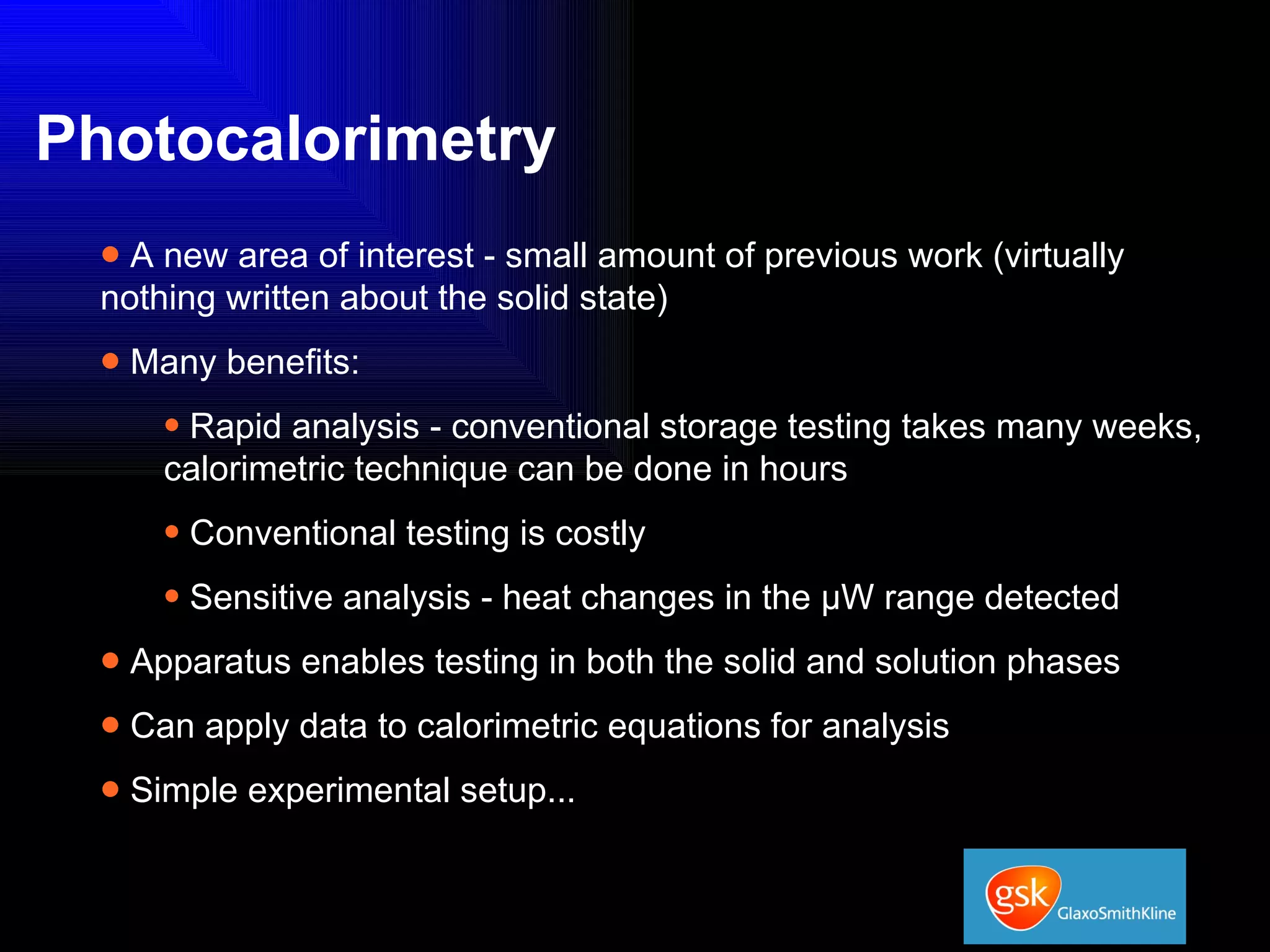
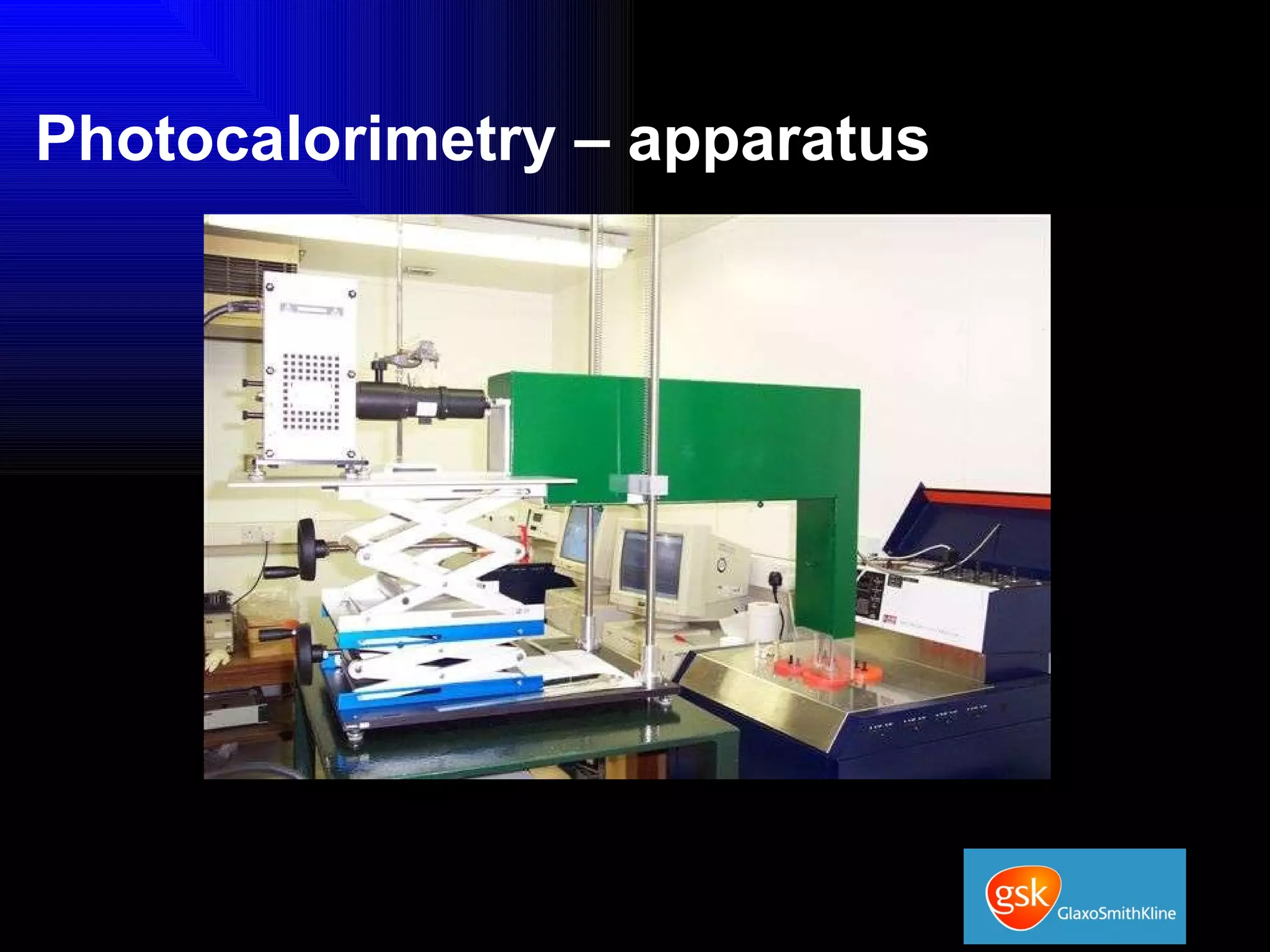
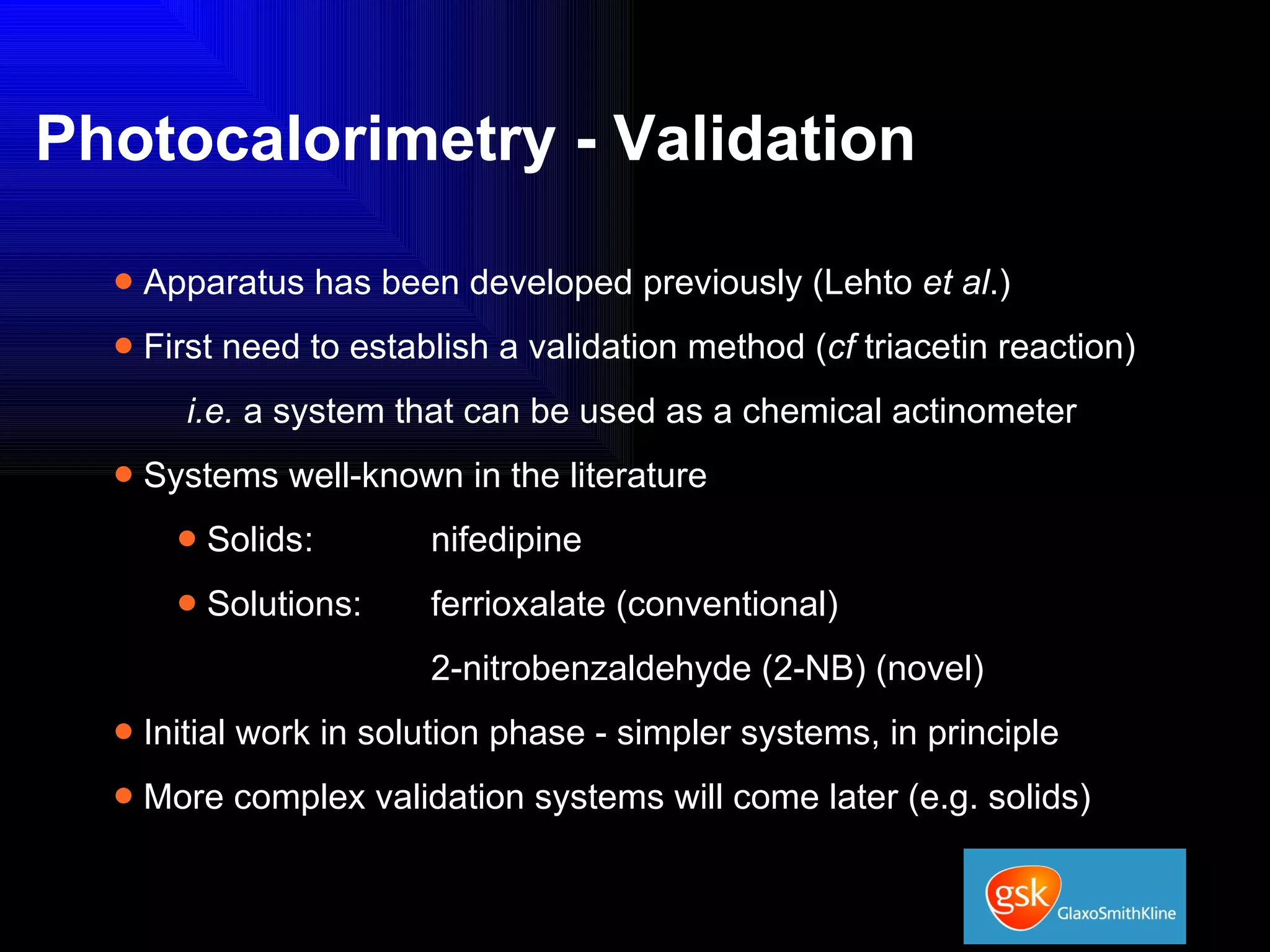
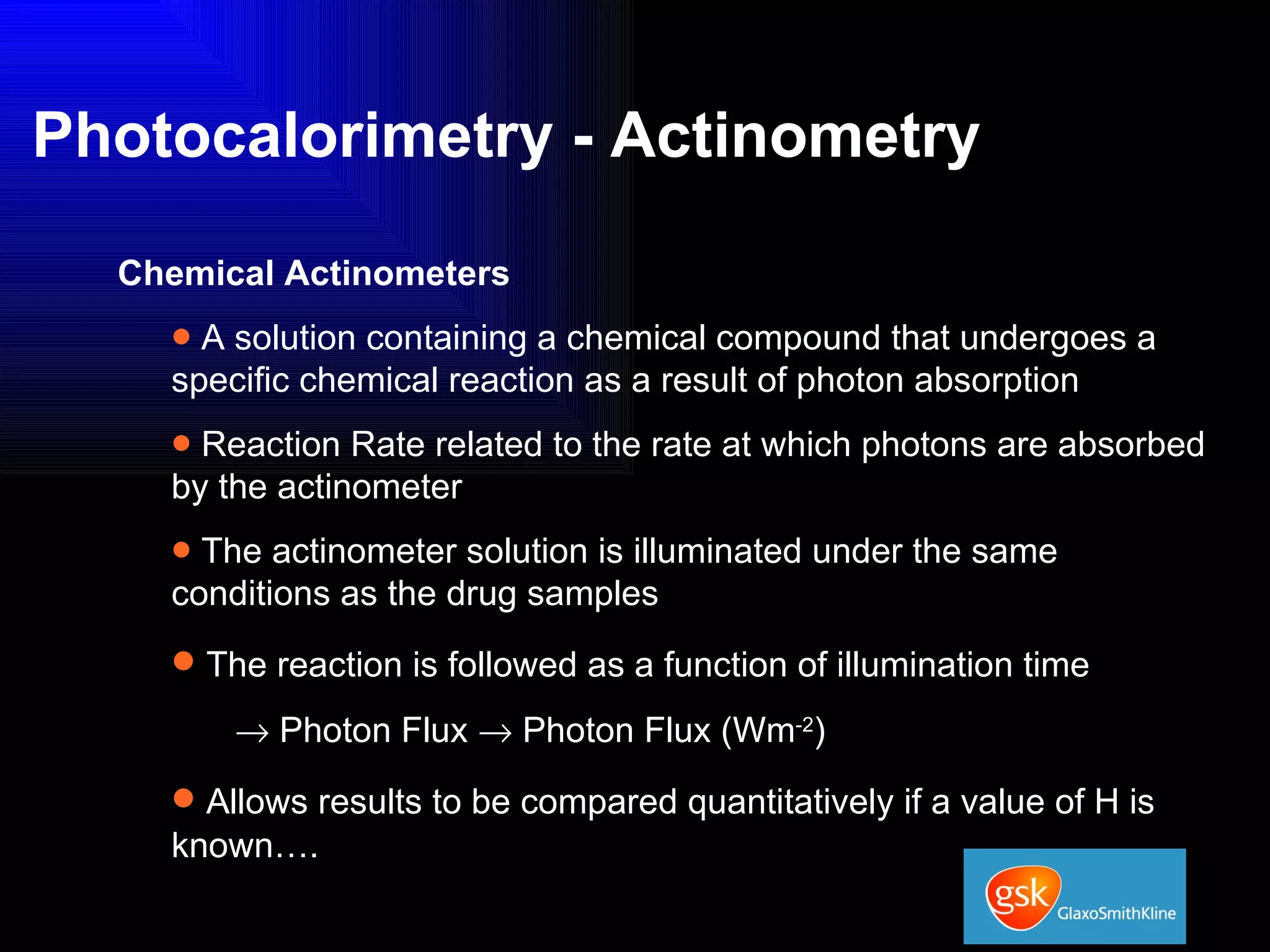
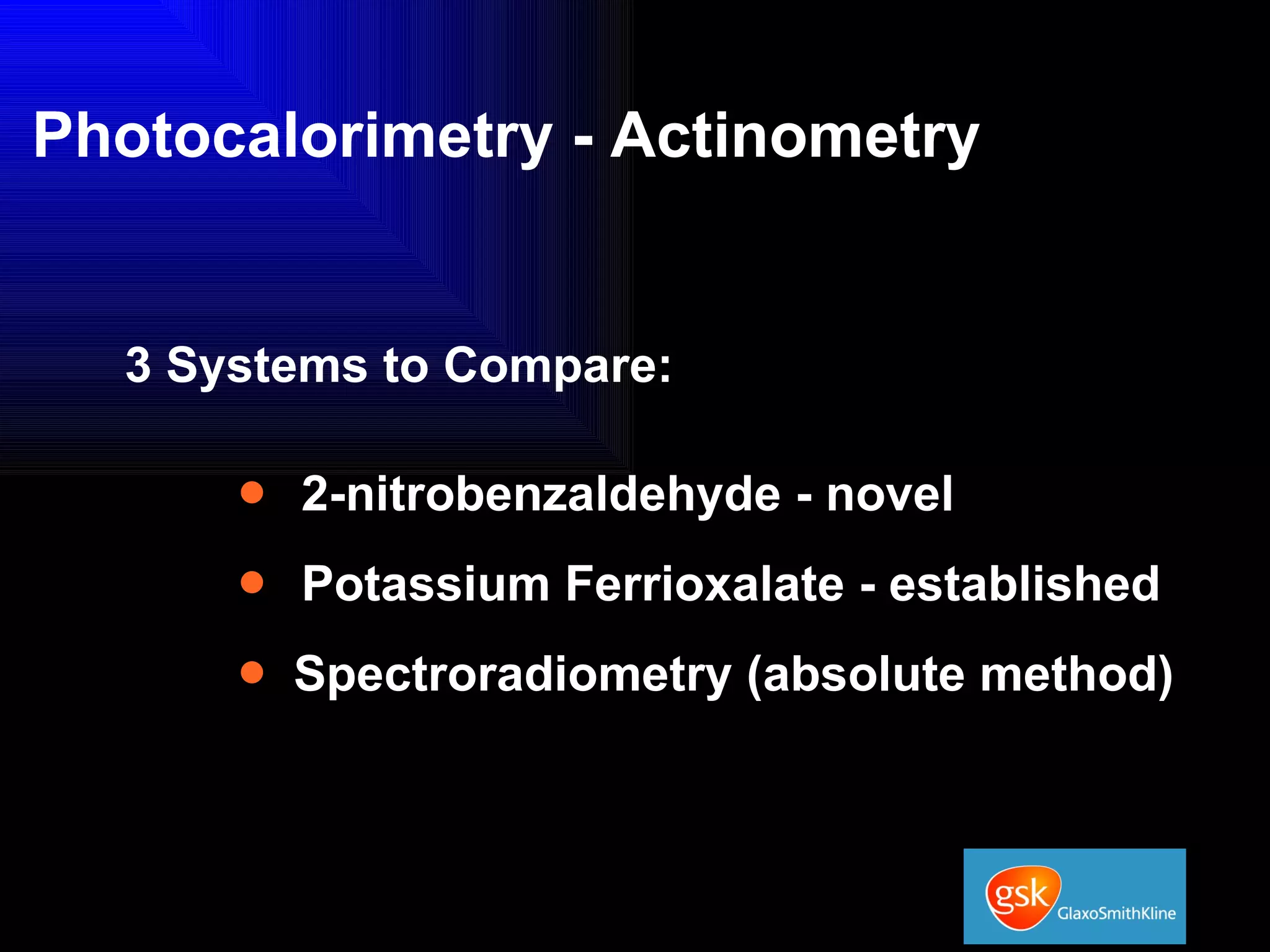
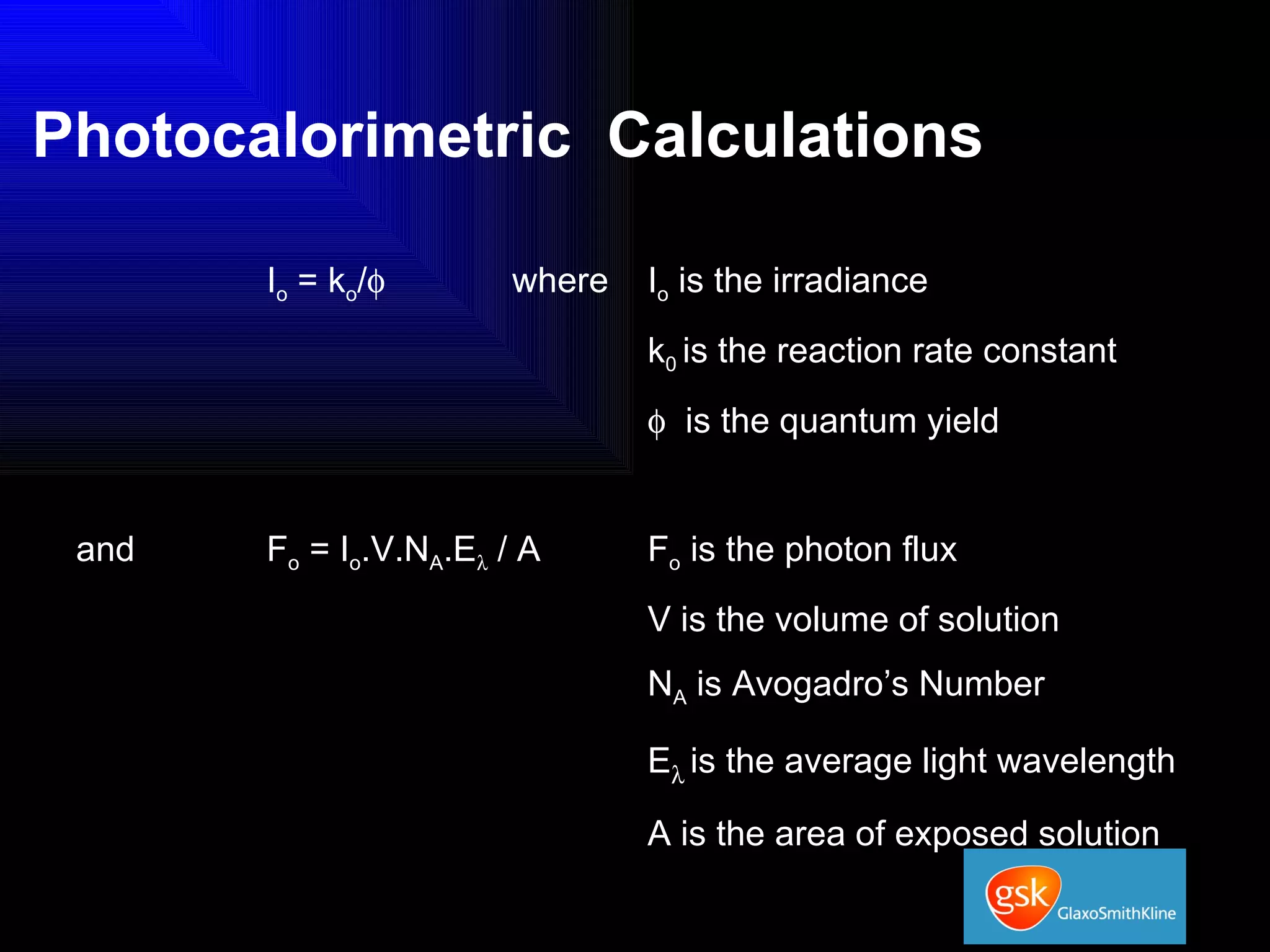
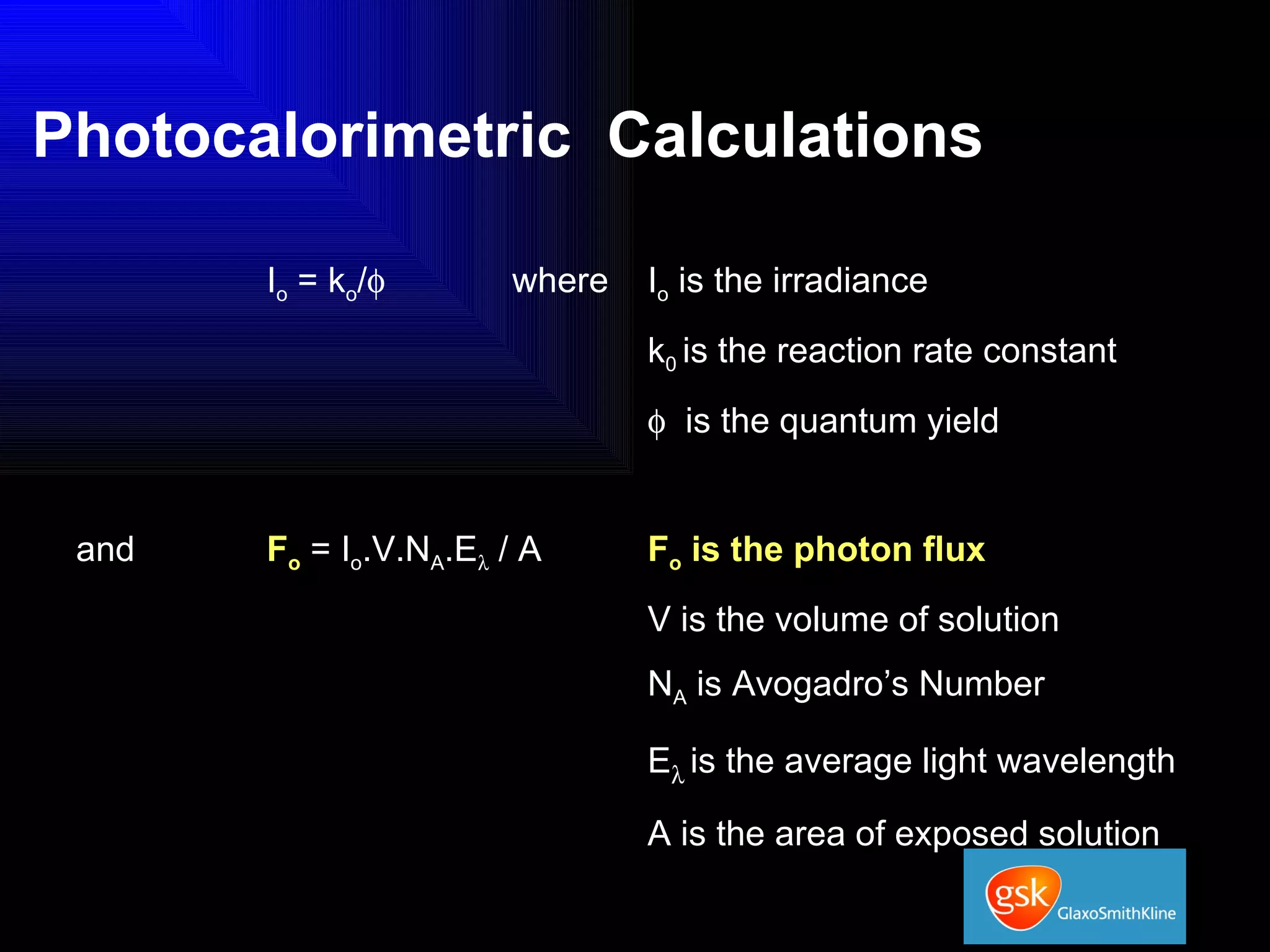
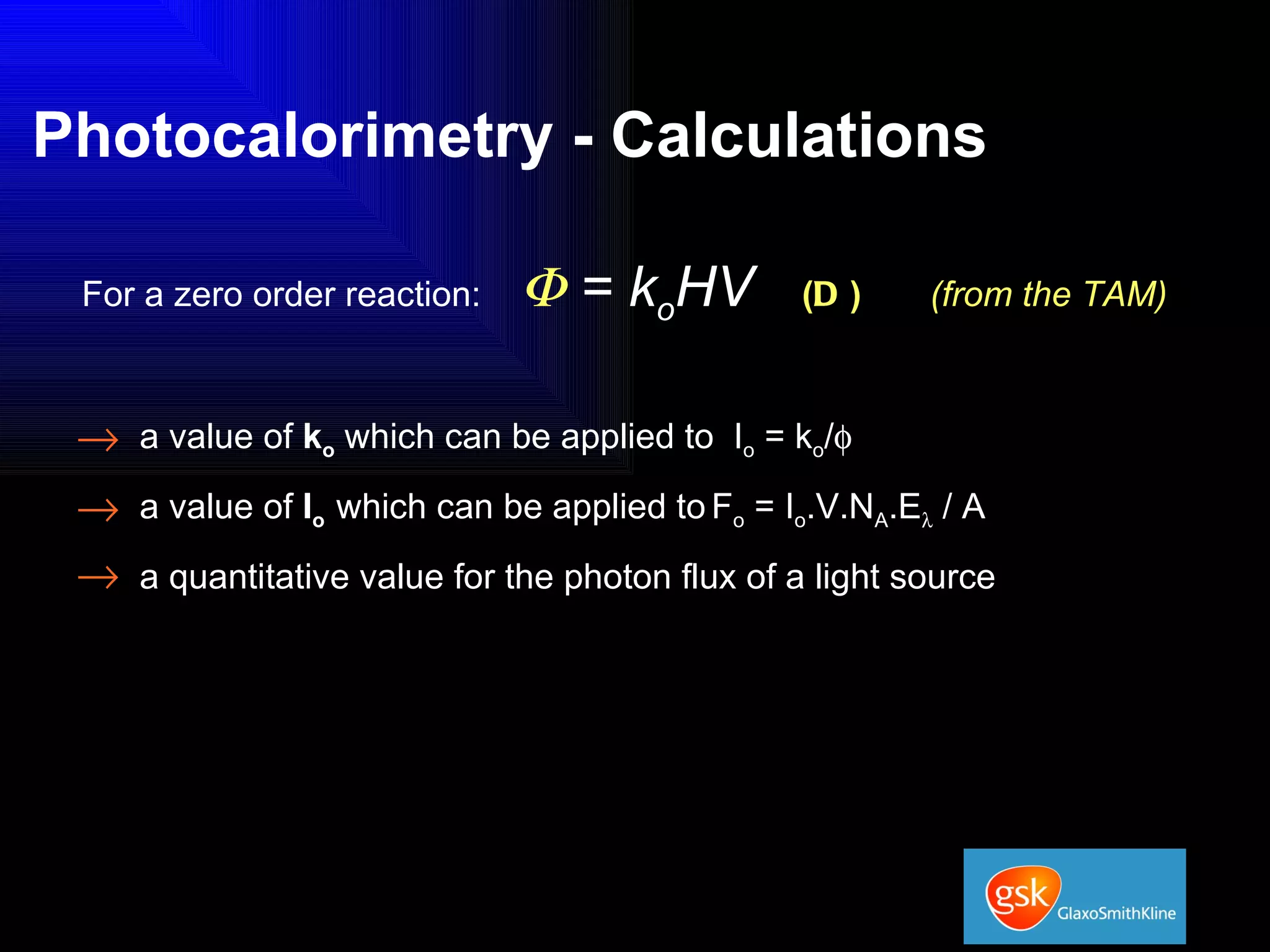
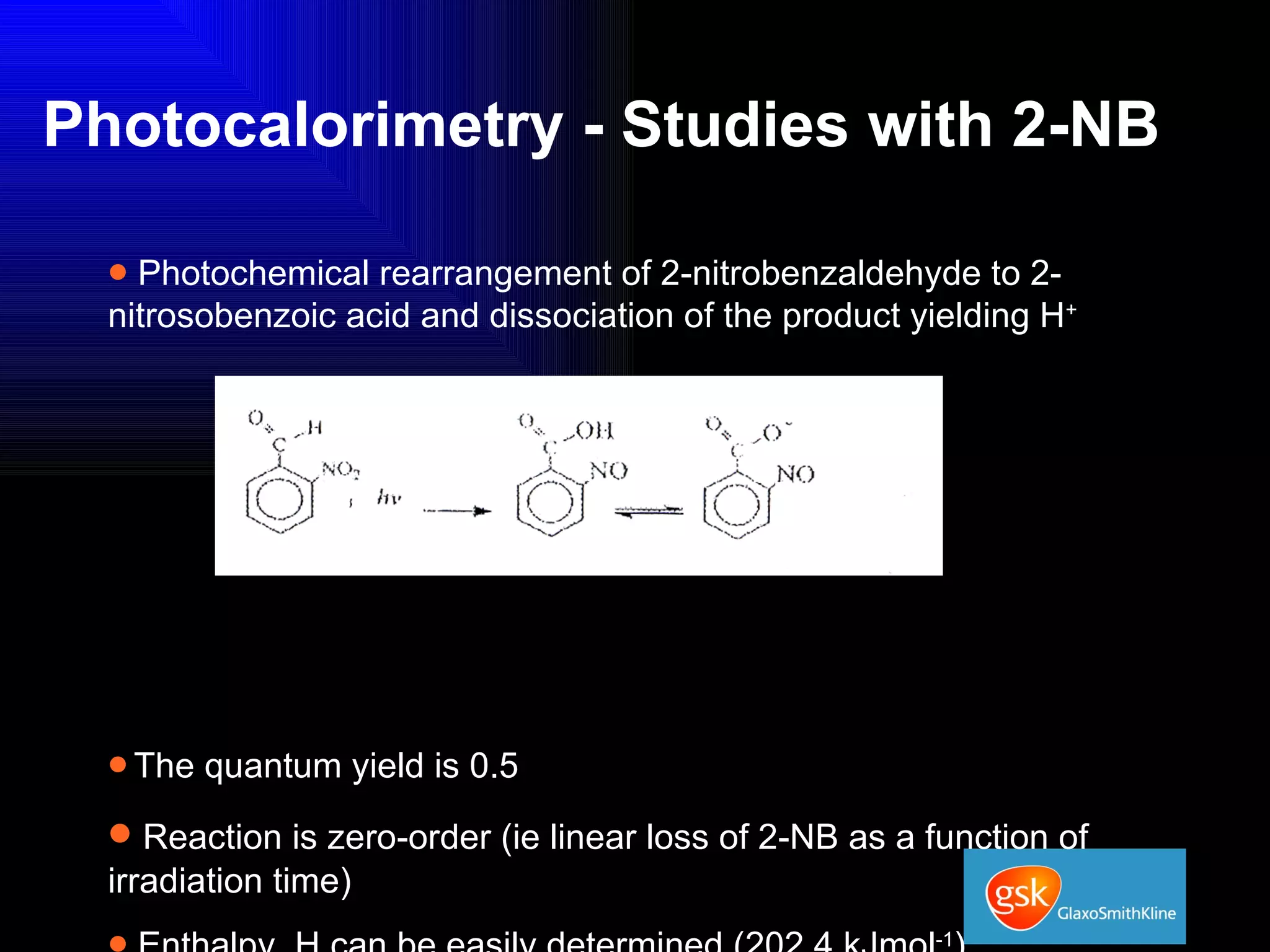
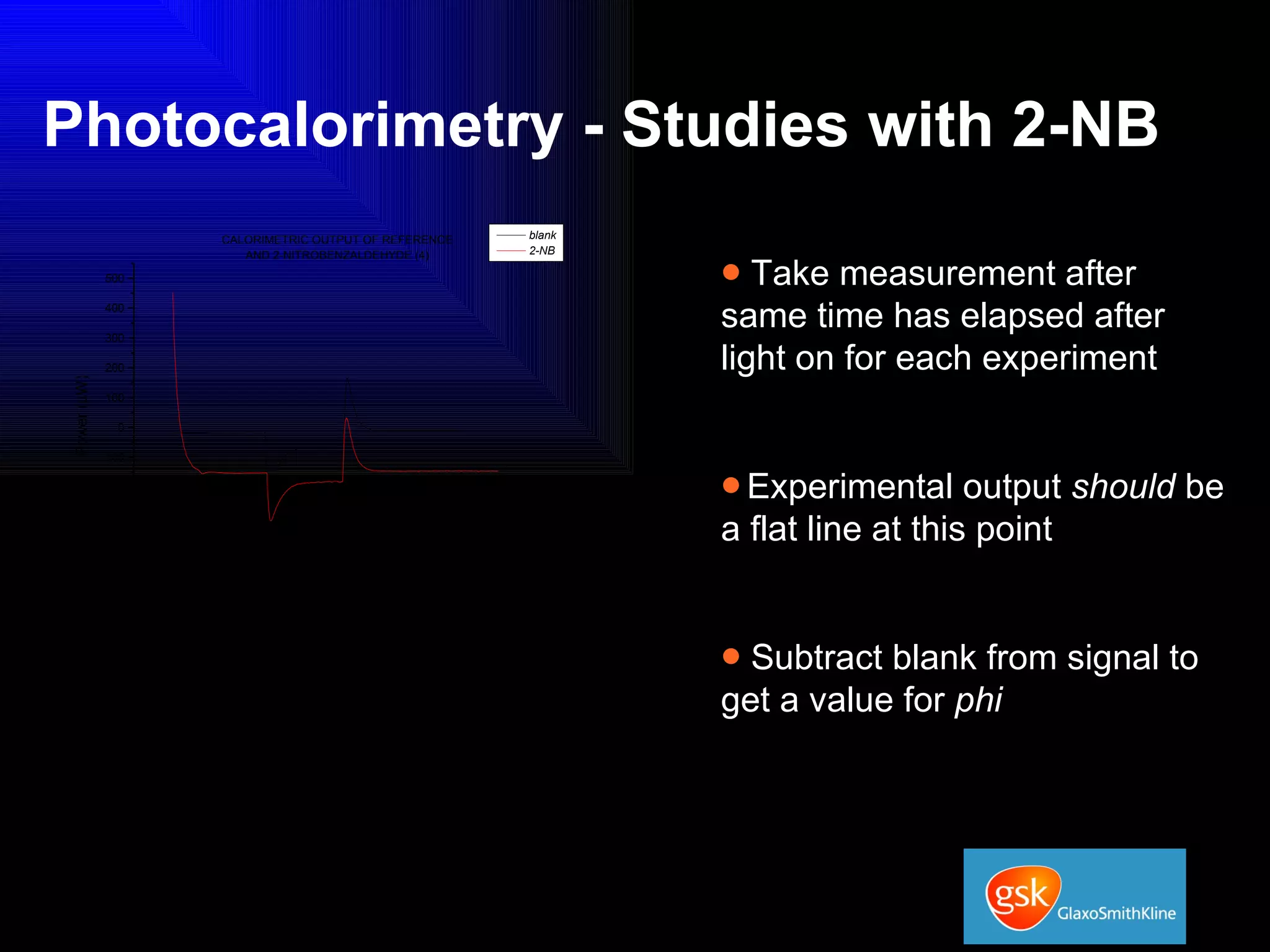
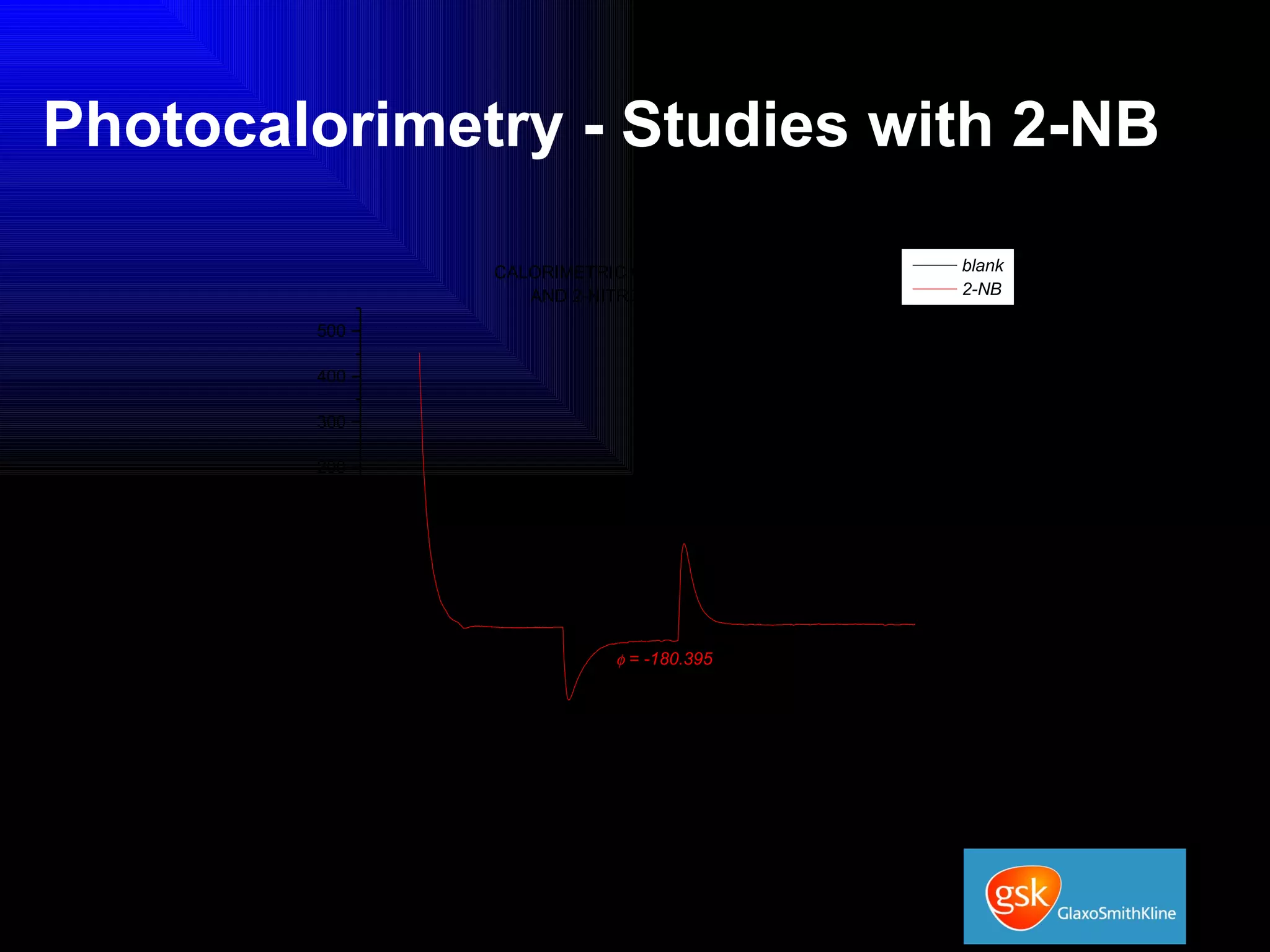
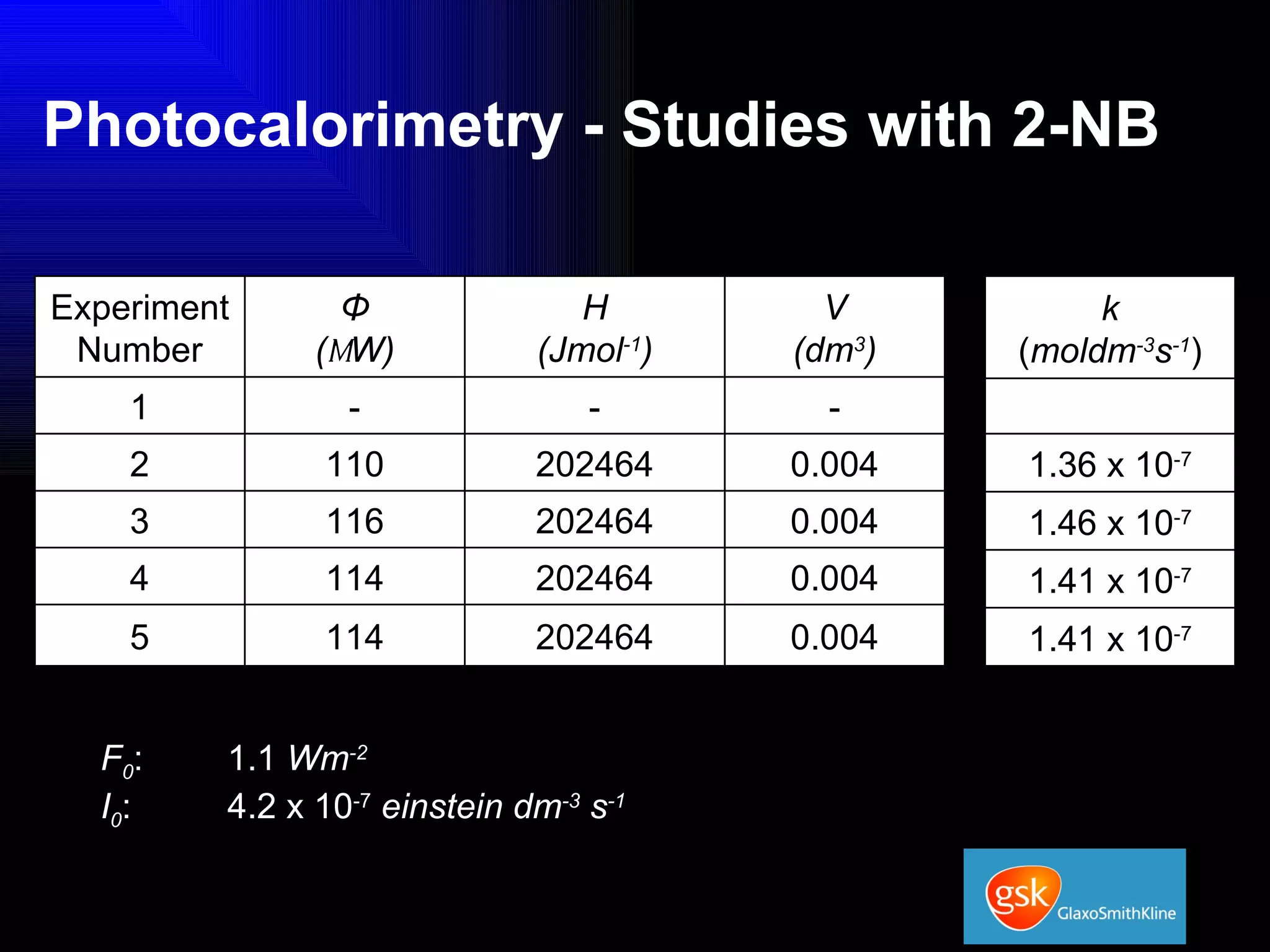
The document summarizes the development and validation of a photocalorimetry technique. It outlines the design of the photocalorimeter instrument and describes validation methods using chemical actinometers like 2-nitrobenzaldehyde and ferrioxalate in solution. Nifedipine degradation experiments were used to validate the technique for solids. Results showed 2-NB provided more stable calibration than ferrioxalate. Future work will apply the technique to study effects of wavelength, moisture and temperature on solid-state reactions.



![Photocalorimetry - Studies with Ferrioxalate Traditional chemical actinometer 2[Fe(C 2 O 4 ) 3 ] 3- 2Fe(C 2 O 4 ) 2- + 2CO 2 Many advantages over 2-NB: 1. Sensitivity 2. Wavelength Coverage 3. Photolyte stability and Photolysis Products 4. Simplicity of operation 5. Has a known enthalpy Highly regarded and widely used Adopt the same principles for calculation as before….](https://image.slidesharecdn.com/tac2004-110113133228-phpapp02/75/Tac-2004-17-2048.jpg)