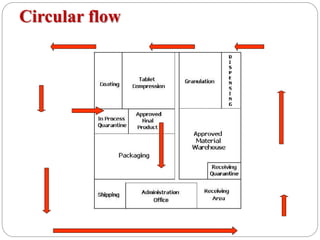
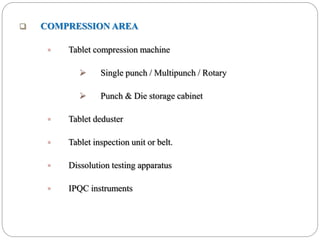
This document discusses the layout, facilities, environmental requirements, and equipment of a pharmaceutical manufacturing facility. It describes the facility as being designed according to GMP practices to prevent cross contamination with proper air handling, cleaning, lighting, and plumbing. The layout is organized with three types: circular flow, parallel flow, and crossover traffic. Environmental controls are needed for product and employee protection including temperature, humidity, and air pressure controls. The equipment listed includes mixing, granulation, drying, compression, coating, and packaging machinery.