This case report describes a patient who underwent multiple cardiac surgeries for transposition of the great arteries with ventricular septal defect. The patient developed recurrent neoaortic insufficiency after an arterial switch operation and subsequent aortic valve replacement. A "switch back" operation was performed, placing the native pulmonary valve back into the left ventricular outflow tract. However, severe aortic insufficiency recurred within a year, requiring another aortic valve replacement. Histopathology of the excised valve showed abnormal elastic tissue, suggesting an underlying structural problem contributed to valve dysfunction. The report questions whether the switch back operation is a viable long-term option for neoaortic insufficiency in patients with complex transposition anatomies.

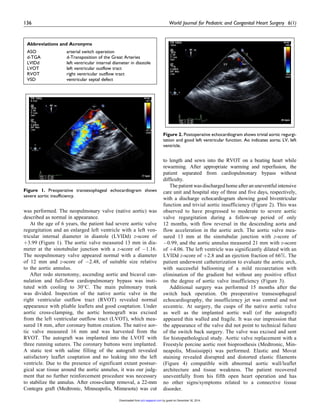
![Comments
Neoaortic valve regurgitation and neoaortic root dilatation are
not uncommon findings after an arterial switch procedure. For-
tunately, surgical intervention is rarely necessary, with free-
dom from reoperation for aortic insufficiency at 10 and
15 years being 97.7% and 96.8%, respectively, when consider-
ing all patients having transposition with or without a VSD.3
In
the uncommon instance where reoperation is required, options
include valve repair, replacement, a Bentall procedure, a valve-
sparing root replacement, or a Konno procedure,2
all of which
have satisfactory results with low mortality. Data suggest that
freedom from reintervention decreases with risk factors such
as male sex and complex d-TGA with VSD.1-2
Bove´ et al found
that a concomitant VSD with associated discrepancy in pul-
monary valve to aortic root size was an important predictor for
valve dysfunction.2
Aortic arch hypoplasia requiring patch
augmentation is also a risk factor for aortic insufficiency due
to increased turbulence leading to increased aortic root dilation
and pressure.2
Our patient had aortic arch hypoplasia and a
VSD, requiring revision of the VSD patch closure seven days
postoperatively due to the proximity of the VSD patch to the
right coronary leaflet of the neoaortic valve. Surgical aortic
valve replacement was required at 17 months of life. The initial
mechanism of neoaortic valve insufficiency could have been
iatrogenic damage to the valve that may have occurred as a
result of manipulation or direct injury associated with closure
of the VSD through the aortic annulus. The neopulmonary
valve (the patient’s original aortic valve) was always documen-
ted as being normal with no evidence of native aortic and pul-
monary valve mismatch, with normal z-scores for both annuli.
After progressive failure of the aortic homograft valve, the
options that were considered included surgical repair, replace-
ment with a mechanical valve, homograft, or xenograft pros-
theses, and the switch back operation. We chose to proceed
with the switch back operation, first described by Hazekamp
et al in 1997,4
combining the advantages of placing the native
aortic valve back in the left ventricular outflow4
with freedom
from lifetime anticoagulation. There is limited literature about
the switch back operation, also called the ‘‘reverse Ross oper-
ation,’’ with a relatively small number of reported cases includ-
ing this case. There is only one other report of the Switch Back
operation after an arterial switch and VSD closure for d-TGA
and VSD by Vicente et al,5
with a good result at five-year fol-
low-up.
Limitations of the switch back procedure include surgical
complexity and possible further need for reoperation, since a
single-valve disease is converted into a condition potentially
affecting two valves, with the likely need to replace the right
ventricle to pulmonary artery conduit in the patient’s future.
The mechanism of recurrent aortic insufficiency is unknown
but could be due to the effect of higher systemic pressure on
what functioned previously as a pulmonary valve,4,6
surgical
technique with a trapdoor coronary artery button reimplanta-
tion, or a larger sized aortic root for both patients with TGA/
intact ventricular septum and TGA/VSD .2,6
Theoretically, aor-
tic insufficiency could be related to afterload associated with
arch obstruction (even of mild degree), a continuous suture
technique during the switch-back autograft implantation or the
prior presence of a VSD with d-TGA with an increased left
ventricular annular z-score (À1.16 in our patient, therefore an
improbable mechanism). In our patient, aortic annulus dilation
may have occurred as a consequence of surgical manipulation
during initial VSD closure through the aortic annulus, which
may have distorted the left ventricular/aortic junction. The
Switch Back operation reported here was initially a success
with trivial aortic insufficiency seen on postoperative
Figure 3. Image from cardiac catheterization 12 months after the
switch back operation demonstrating recurrent severe aortic valve
insufficiency.
Figure 4. Distorted and disrupted elastica is seen on the aortic side of
the aortic valve leaflet (right; elastica [Â10 original magnification]).
Brister et al 137
by guest on December 30, 2014pch.sagepub.comDownloaded from](https://image.slidesharecdn.com/66e8de56-6fa8-4498-b7a5-a2432d781f09-161218181658/85/Switch-back-reverse-Ross-WJPCHS-3-320.jpg)
